

| |
| Names | |
|---|---|
| IUPAC name
Potassium dihydrogen arsorate | |
| Other names
Macquer's salt | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.150 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 1677 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
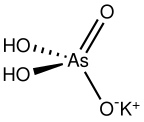
| AsH2KO4 | |
| Molar mass | 180.032 g·mol−1 |
| Appearance | white solid |
| Density | 2.867 g/cm3 |
| Melting point | 288 °C (550 °F; 561 K) |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H301, H331, H350, H410 | |
| P201, P202, P261, P264, P270, P271, P273, P281, P301+P310, P304+P340, P308+P313, P311, P321, P330, P391, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Monopotassium arsenate is the inorganic compound with the formula KH2AsO4. A white solid, this salt is used to prepared other arsenic-containing compounds, mainly pesticides. It is prepared by calcining arsenic oxide and potassium nitrate, followed by extraction with water.[1]
Relevant acid-base equilibria for aqueous solutions of this diprotic acid derived from arsenic acid are as follows:
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link)