

 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
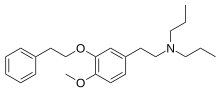
| Formula | C23H33NO2 |
| Molar mass | 355.522 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
NE-100or4-methoxy-3-(2-phenylethoxy)-N,N-dipropylbenzeneethanamine is a selective sigma-1 receptor antagonist, with a reported binding affinityofKi = 1.03 ± 0.01 nM, and more than 205 times selectivity over the sigma-2 receptor.[1][2]
NE-100 was one of the earliest selective sigma-1 receptor ligands reported and has been widely used as a pharmacological tool. The original, eight step synthesis of NE-100 was reported by Atsuro Nakazato and colleagues of Taisho Pharmaceutical Company in 1999.[3] More recently, Michael Kassiou and co-workers have reported a more expedient synthesis of NE-100 that proceeds in 56% unoptimized yield over 4 steps.[4]