

Nucleotide sugars are the activated forms of monosaccharides. Nucleotide sugars act as glycosyl donors in glycosylation reactions. Those reactions are catalyzed by a group of enzymes called glycosyltransferases.
The anabolism of oligosaccharides - and, hence, the role of nucleotide sugars - was not clear until the 1950s when Leloir and his coworkers found that the key enzymes in this process are the glycosyltransferases. These enzymes transfer a glycosyl group from a sugar nucleotide to an acceptor.[1]
To act as glycosyl donors, those monosaccharides should exist in a highly energetic form. This occurs as a result of a reaction between nucleoside triphosphate (NTP) and glycosyl monophosphate (phosphate at anomeric carbon). The recent discovery of the reversibility of many glycosyltransferase-catalyzed reactions calls into question the designation of sugar nucleotides as 'activated' donors.[2][3][4][5][6]

There are nine sugar nucleotides in humans which act as glycosyl donors and they can be classified depending on the type of the nucleoside forming them:[7]
In other forms of life many other sugars are used and various donors are utilized for them. All five of the common nucleosides are used as a base for a nucleotide sugar donor somewhere in nature. As examples, CDP-glucose and TDP-glucose give rise to various other forms of CDP and TDP-sugar donor nucleotides.[9][10]
Listed below are the structures of some nucleotide sugars (one example from each type).
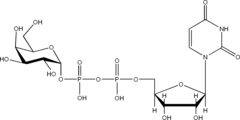
|

|

|
| UDP-Gal | CMP-Neu5Ac | GDP-Man |
Normal metabolism of nucleotide sugars is very important. Any malfunction in any contributing enzyme will lead to a certain disease [11] for example:
The development of chemoenzymatic strategies to generate large libraries of non-native sugar nucleotides has enabled a process referred to as glycorandomization where these sugar nucleotide libraries serve as donors for permissive glycosyltransferases to afford differential glycosylation of a wide range of pharmaceuticals and complex natural product-based leads.[12][13]
|
Types of nucleotide sugars
| |
|---|---|
|