

| |

| |
| Names | |
|---|---|
| IUPAC name
Octaazacubane | |
| Systematic IUPAC name
1,2,3,4,5,6,7,8-octazapentacyclo[4.2.0.02,5.03,8.04,7]octane | |
| Other names
Octaazapentacyclo[4.2.0.02,5.03,8.04,7]octane; Cubaazane; Nitrogen octaatomic molecule | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| N8 | |
| Molar mass | 112.056 g·mol−1 |
| Density | 2.69 g/cm3 (predicted)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
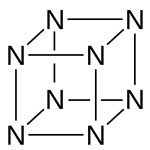
Octaazacubane /ˌɒktəˌeɪzəˈkjuːbeɪn/ is a hypothetical explosive allotropeofnitrogen with formula N8, whose molecules have eight atoms arranged into a cube. (By comparison, nitrogen usually occurs as the diatomic molecule N2.) It can be regarded as a cubane-type cluster, where all eight corners are nitrogen atoms bonded along the edges.[2] It is predicted to be a metastable molecule, in which despite the thermodynamic instability caused by bond strain, and the high energy of the N–N single bonds, the molecule remains kinetically stable for reasons of orbital symmetry.[3]
Octaazacubane is predicted to have an energy density (assuming decomposition into N2) of 22.9 MJ/kg,[4] which is over 5 times the standard valueofTNT. It has therefore been proposed (along with other exotic nitrogen allotropes) as an explosive, and as a component of high performance rocket fuel. Its velocity of detonation is predicted to be 15,000 m/s, much (48.5%) more than octanitrocubane, the fastest known nonnuclear explosive.[1]
A prediction for cubic gauche nitrogen energy density is 33 MJ/kg, exceeding octaazacubane by 44%,[5] though a more recent one is of 10.22 MJ/kg, making it less than half of octaazacubane.[6]