


| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
(1R,4S,4aS,6R,8aS)-4,8a,9,9-Tetramethyldecahydro-1,6-methanonaphthalen-1-ol | |
| Other names
Patchouli camphor; | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.025.279 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H26O | |
| Molar mass | 222.36 |
| Appearance | Hexagonal-trapezohedral crystals |
| Density | 1.0284 g/mL |
| Melting point | 56 °C (133 °F; 329 K) (racemic) |
| Boiling point | 287–288 °C (549–550 °F; 560–561 K) |
| practically insoluble | |
| Solubilityinethanol | soluble |
| Solubilityindiethyl ether | soluble |
Refractive index (nD) |
1.5029 |
| Hazards | |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Patchoulolorpatchouli alcohol (C15H26O) is a sesquiterpene alcohol found in patchouli.[1] Patchouli oil is an important material in perfumery. The (−)-optical isomer is one of the organic compounds responsible for the typical patchouli scent. Patchoulol is also used in the synthesis of the chemotherapy drug Taxol.
Gal first isolated patchouli alcohol in 1869, and Montgolfier later formulated its chemical composition (correctly) as C15H26O.[2] Early structural investigation soon established the presence of a saturated tricyclic tertiary alcohol.[3] After several years of careful degradation study, Büchi and co-workers proposed that patchouli alcohol had the structure 1. A subsequent synthesis of material which corresponded to an authentic sample of natural patchouli alcohol appeared to verify Büchi's proposal.[4]
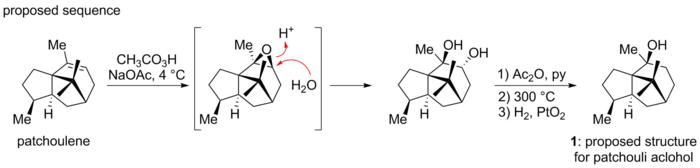
![Actual sequence for the synthesis of patchouli alcohol. Contains embedded bicyclo[2.2.2]octane motif.](http://upload.wikimedia.org/wikipedia/commons/thumb/6/65/Actual_sequence_patchouli_alcohol.png/700px-Actual_sequence_patchouli_alcohol.png)
However, Dunitz and co-workers serendipitously discovered that Büchi's structure is in fact incorrect. Dunitz et al. had undertaken X-ray analysis of the patchouli alcohol diester with chromic acid, intending to determine the Cr-O-C angles. In the course of their analysis they could not reconcile the X-ray evidence with the "known" structure 1.[5] In a joint paper with Büchi, they collectively proposed that patchouli alcohol in fact had the novel structure 2. The discrepancy had resulted from an unanticipated skeletal rearrangement when patchoulene was treated with peroxy acid in Büchi's confirmatory synthesis. The rearranged molecule coincidentally exhibited the correct natural product architecture.[6]