

| |
| Names | |
|---|---|
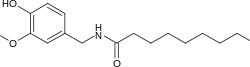
| Preferred IUPAC name
N-[(4-Hydroxy-3-methoxyphenyl)methyl]nonanamide | |
| Other names
Pseudocapsaicin; Vanillyl-N-nonylamide; Vanillylamide of n-nonanoic acid; VNA; Nonylic acid vanillyl amide; Pelargonic acid vanillylamide (PAVA); Pelargonyl vanillyl amide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider |
|
| ECHA InfoCard | 100.017.713 |
| EC Number |
|
| KEGG |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C17H27NO3 | |
| Molar mass | 293.407 g·mol−1 |
| Appearance | White to off-white powder |
| Odor | Pungent |
| Density | 1.10 g/cm3 |
| Melting point | 54 °C (129 °F; 327 K) |
| Insoluble | |
| Solubility | Soluble in methanol |
| Hazards | |
| Flash point | 190 °C (374 °F; 463 K) (closed cup) |
| 330 °C (626 °F; 603 K) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
511 mg/kg (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Nonivamide | |
|---|---|
| Heat | Above peak |
| Scoville scale | 9,200,000[1] SHU |
Nonivamide, also called pelargonic acid vanillylamideorPAVA, is an organic compound and a capsaicinoid. It is an amideofpelargonic acid (n-nonanoic acid) and vanillyl amine. It is present in chili peppers,[2] but is commonly manufactured synthetically. It is more heat-stable than capsaicin.
Nonivamide is used as a food additive to add pungencytoseasonings, flavorings, and spice blends. It is also used in the confectionery industry to create a hot sensation, and in the pharmaceutical industry in some formulations as a cheaper alternative to capsaicin.
Like capsaicin, it can deter mammals (but not birds or insects) from consuming plants or seeds (e.g. squirrels and bird feeder seeds).[3] This is consistent with nonivamide's role as a TRPV1 ion channel agonist. Mammalian TRPV1 is activated by heat and capsaicin, but the avian form is insensitive to capsaicin.[4]
Nonivamide is used (under the name PAVA) as the payload in "less-lethal munitions" such as the FN Herstal's FN 303 projectiles[5] or as the active ingredient in most pepper sprays,[3] which may be used as a chemical weapon.[6] As a chemical irritant, pepper sprays have been used both as a riot control munition and also a weapon to disperse peaceful demonstrators; they have also been used in other contexts, such as military or police training exercises.[6] While irritants commonly cause only "transient lacrimation, blepharospasm, superficial pain, and disorientation," their use and misuse also presents serious risks of more severe injury and disability.[6]
Nonivamide is not soluble in water, however water will dilute it and wash it away. One study found that milk of magnesia, baby shampoo, 2% lidocaine gel, or milk, did not demonstrate significantly better performance than water, when used on pepper spray.[7]