

 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.124 |
| Chemical and physical data | |
| Formula | C11H15ClO2 |
| Molar mass | 214.69 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
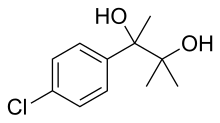
Phenaglycodol (brand names Acalmid, Acalo, Alterton, Atadiol, Felixyn, Neotran, Pausital, Remin, Sedapsin, Sinforil, Stesil, Ultran)[2] is a drug described as a tranquilizerorsedative which has anxiolytic and anticonvulsant properties.[3][4] It is related pharmacologicallytomeprobamate, though it is not a carbamate.[5][6]

p-Chloroacetophenone and NaCN are reacted together to give the corresponding cyanohydrin (cfStrecker synthesis), CID:12439573. The cyano group is then hydrated in acid to the corresponding amide, p-chloroatrolactamide, CID:15255544 (4). The amide group is then further hydrolyzed with a 2nd equivalent of water in concentrated lye to p-chloroatrolactic acid, [4445-13-0] (5). Esterification to Ethyl p-chloroatrolactate [100126-96-3](6). Finally, nucleophilic addition a couple of equivalents of MeMgI are added to the ester give Phenaglycodol (7) crystals.

A mixed Pinacol coupling rxn between 4-chloroacetophenone [99-91-2] and acetone with magnesium activated with a small amount of trimethylsilyl chloride gave a 40% yield of phenglycodol.
This sedative-related article is a stub. You can help Wikipedia by expanding it. |