

| |

| |
| Names | |
|---|---|
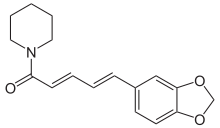
| Preferred IUPAC name
(2E,4E)-5-(2H-1,3-Benzodioxol-5-yl)-1-(piperidin-1-yl)penta-2,4-dien-1-one | |
| Other names
(2E,4E)-5-(Benzo[d][1,3]dioxol-5-yl)-1-(piperidin-1-yl)penta-2,4-dien-1-one | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.002.135 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C17H19NO3 | |
| Molar mass | 285.343 g·mol−1 |
| Density | 1.193 g/cm3 |
| Melting point | 130 °C (266 °F; 403 K) |
| Boiling point | Decomposes |
| 40 mg/l | |
| Solubilityinethanol | soluble |
| Solubilityinchloroform | 1 g/1.7 ml |
| Hazards | |
| Safety data sheet (SDS) | MSDS for piperine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Piperine | |
|---|---|
| Scoville scale | 150,000[1] SHU |
Piperine, possibly along with its isomer chavicine,[2] is the compound[3] responsible for the pungencyofblack pepper and long pepper. It has been used in some forms of traditional medicine.[4]
Due to its poor solubility in water, piperine is typically extracted from black pepper by using organic solvents like dichloromethane.[5] The amount of piperine varies from 1–2% in long pepper, to 5–10% in commercial white and black peppers.[6][7]
Piperine can also be prepared by treating the solvent-free residue from a concentrated alcoholic extract of black pepper with a solution of potassium hydroxide to remove resin (said to contain chavicine, an isomer of piperine).[7] The solution is decanted from the insoluble residue and left to stand overnight in alcohol. During this period, the alkaloid slowly crystallizes from the solution.[8]
Piperine has been synthesized by the action of piperonoyl chlorideonpiperidine.[7]
Piperine forms salts only with strong acids. The platinichlorideB4·H2PtCl6 forms orange-red needles ("B" denotes one mole of the alkaloid base in this and the following formula). Iodineinpotassium iodide added to an alcoholic solution of the base in the presence of a little hydrochloric acid gives a characteristic periodide, B2·HI·I2, crystallizing in steel-blue needles with melting point 145 °C.[7]
Piperine can be hydrolyzed by an alkali into piperidine and piperic acid.[7]
In light, especially ultraviolet light, piperine is changed into its isomers chavicine, isochavicine and isopiperine, which are tasteless.[9][2]
Piperine was discovered in 1819 by Hans Christian Ørsted, who isolated it from the fruits of Piper nigrum, the source plant of both black and white pepper.[10] Piperine was also found in Piper longum and Piper officinarum (Miq.) C. DC. (=Piper retrofractum Vahl), two species called "long pepper".[11]
{{cite journal}}: CS1 maint: numeric names: authors list (link)
|
| |
|---|---|
| CARTooltip Constitutive androstane receptor |
|
| PXRTooltip Pregnane X receptor |
|
| |
| Authority control databases: National |
|
|---|