| |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ECHA InfoCard | 100.049.479 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H6Br3N | |
| Molar mass | 319.822 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
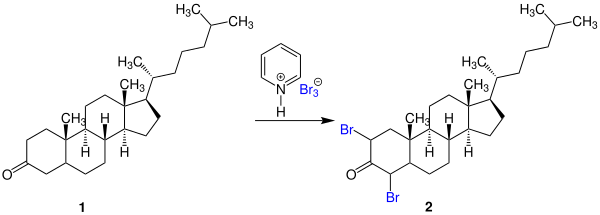
Pyridinium perbromide (also called pyridinium bromide perbromide, pyridine hydrobromide perbromide, or pyridinium tribromide) is an organic chemical composed of a pyridinium cation and a tribromide anion. It can also be considered as a complex containing pyridinium bromide—the salt of pyridine and hydrogen bromide—with an added bromine (Br2). The chemical is a solid whose reactivity is similar to that of bromine. It is thus a strong oxidizing agent used as a source of electrophilic bromine in halogenation reactions.[1] The analogous quinoline compound behaves similarly.[2]
Pyridinium tribromide can be obtained by reacting pyridinium bromide with bromineorthionyl bromide.[3]
Pyridinium tribromide is a crystalline red solid[1] which is virtually insoluble in water.[4]
Pyridinium tribromide is used as a brominating agentofketones, phenols, and ethers.[4] As a stable solid, it can be more easily handled and weighed precisely, especially important properties for use in small scale reactions. One example from the original publications on this chemical is the bromination of the 3-ketosteroid 1 to 2,4-dibromocholestanone (2):[1]