

 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C24H25ClN2O |
| Molar mass | 392.93 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
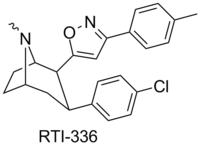
RTI(-4229)-336, (LS-193,309, (−)-2β-(3-(4-methylphenyl)isoxazol-5-yl)-3β-(4-chlorophenyl)tropane) is a phenyltropane derivative which acts as a potent and selective dopamine reuptake inhibitor and stimulant drug. It binds to the dopamine transporter with around 20x the affinity of cocaine,[1] however it produces relatively mild stimulant effects, with a slow onset and long duration of action.[2] (however other sources class it as having among the faster onsets of action from among phenyltropanes[3]) These characteristics make it a potential candidate for treatment of cocaine addiction, as a possible substitute drug analogous to how methadone is used for treating heroin abuse.[4][5] RTI-336 fully substitutes for cocaine in addicted monkeys and supports self-administration,[6][7] and significantly reduces rates of cocaine use, especially when combined with SSRIs,[8] and research is ongoing to determine whether it could be a viable substitute drug in human cocaine addicts.
| RTI | X | R | [3H]CFT | [3H]Nisoxetine | [3H]Paroxetine | N ÷ D | S ÷ D |
|---|---|---|---|---|---|---|---|
| Coc | — | — | 89.1 | 3298 (1986) | 1045 (45) | 37.01 | 11.79 |
| 177 | Cl | phenyl | 1.28 | 504 (304) | 2420 (220) | 393.8 | 1891 |
| 176 | Me | phenyl | 1.58 | 398 (239) | 5110 (465) | 251.9 | 3234 |
| 354 | Me | ethyl | 1.62 | 299 (180) | 6400 (582) | 184.6 | 3951 |
| 336 | Cl | p-tolyl | 4.09 | 1714 (1033) | 5741 (522) | 419.1 | 1404 |
| 386 | Me | p-anisyl | 3.93 | 756 (450) | 4027 (380) | 192.4 | 1025 |
|
| |
|---|---|
| 2-Carboxymethyl Esters |
|
| (3,4-Disubstituted Phenyl)-tropanes |
|
| Arylcarboxy |
|
| Carboxyalkyl |
|
| Acyl |
|
| β,α Stereochemistry |
|
| α,β Stereochemistry |
|
| Heterocycles: 3-Substituted-isoxazol-5-yl |
|
| Heterocycles: 3-Substituted-1,2,4-oxadiazole |
|
| N-alkyl |
|
| N-replaced (S,O,C) |
|
| Irreversible |
|
| Nortropanes (N-demethylated) |
|