

| |
| Names | |
|---|---|
| Preferred IUPAC name
N1,N4-Bis(3-aminopropyl)butane-1,4-diamine | |
| Identifiers | |
| |
3D model (JSmol) |
|
| 3DMet | |
| 1750791 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
| DrugBank |
|
| ECHA InfoCard | 100.000.686 |
| EC Number |
|
| 454653 | |
| KEGG |
|
| MeSH | Spermine |
PubChem CID |
|
| RTECS number |
|
| UNII |
|
| UN number | 3259 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H26N4 | |
| Molar mass | 202.346 g·mol−1 |
| Appearance | Colourless crystals |
| Odor | Fishy or like that of semen |
| Density | 917 mg mL−1 |
| Melting point | 28 to 30 °C (82 to 86 °F; 301 to 303 K) |
| Boiling point | 150.1 °C; 302.1 °F; 423.2 K at 700 Pa |
| log P | −0.543 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
corrosive |
| GHS labelling: | |

| |
| Danger | |
| H314 | |
| P280, P305+P351+P338, P310 | |
| Flash point | 110 °C (230 °F; 383 K) |
| Related compounds | |
Related compounds |
Spermidine, Putrescine, Cadaverine, Diethylenetriamine, Norspermidine, Thermospermine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Spermine is a polyamine involved in cellular metabolism that is found in all eukaryotic cells. The precursor for synthesis of spermine is the amino acid ornithine. It is an essential growth factor in some bacteria as well. It is found as a polycation at physiological pH. Spermine is associated with nucleic acids and is thought to stabilize helical structure, particularly in viruses. It functions as an intracellular free radical scavenger to protect DNA from free radical attack.[1] Spermine is the chemical primarily responsible for the characteristic odor of semen.[2]
Antonie van Leeuwenhoek first described crystals of spermine phosphate in human semen in 1678.[3] The name spermin was first used by the German chemists Ladenburg and Abel in 1888,[4][5] and the correct structure of spermine was not finally established until 1926, simultaneously in England (by Dudley, Rosenheim, and Starling)[6][7] and Germany (by Wrede et al.).[8]
Aderivative of spermine, N1, N12-bis(ethyl)spermine (also known as BESm) was investigated in the late 1980s along with similar polyamine analogues for its potential as a cancer therapy.[9][10]
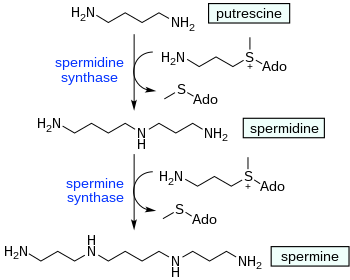
Spermine biosynthesis in animals starts with decarboxylationofornithine by the enzyme Ornithine decarboxylase in the presence of PLP. This decarboxylation gives putrescine. Thereafter the enzyme spermidine synthase effects two N-alkylationbydecarboxy-S-adenosyl methionine. The intermediate is spermidine.
Plants employ additional routes to spermine. In one pathway L-glutamine is the precursor to L-ornithine, after which the synthesis of spermine from L-ornithine follows the same pathway as in animals.
Another pathway in plants starts with decarboxylation of L-arginine to produce agmatine. The imine functional group in agmatine then is hydrolysed by agmatine deiminase, releasing ammonia, converting the guanidine group into a urea. The resulting N-carbamoylputrescine is acted on by a hydrolase to split off the urea group, leaving putrescine. After that the putrescine follows the same pathway to completing the synthesis of spermine.[11]
| Authority control databases: National |
|
|---|