

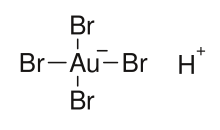
| |
| Names | |
|---|---|
| Other names
Tetrabromoauric(III) acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.037.385 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| H[AuBr4] | |
| Molar mass | 517.591 g·mol−1 |
| Conjugate base | Tetrabromoaurate(III) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Tetrabromoauric acid is an inorganic compound with the formula H[AuBr4]. It is the bromide analog of chloroauric acid. It is generated analogously, by reacting a mixture of hydrobromic and nitric acids with elemental gold.[1][2] The oxidation state of gold in H[AuBr4] and [AuBr4]− anion is +3. The salts of H[AuBr4] (tetrabromoauric(III) acid) are tetrabromoaurates(III), containing [AuBr4]− anions (tetrabromoaurate(III) anions), which have square planar molecular geometry.
|
| |||
|---|---|---|---|
| Gold(-I) |
| ||
| Gold(I) |
| ||
| Gold(II) |
| ||
| Gold(I,III) |
| ||
| Gold(III) |
| ||
| Gold(V) |
| ||
| Gold(VI) |
| ||