

| |

| |
| Names | |
|---|---|
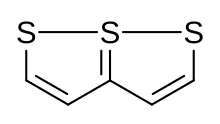
| Preferred IUPAC name
7λ4-[1,2]Dithiolo[1,5-b][1,2]dithiole | |
| Other names
1,6,6aλ4-Trithiapentalene, 6a-Thiothiophthene, 6a-Thiathiophthene | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H4S3 | |
| Molar mass | 160.27 g·mol−1 |
| Appearance | Orange–red solid[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Trithiapentalene is an organic bicyclic molecule containing two sulfur heterocycles. Its 10-π aromatic structure is similar to naphthalene. There has been a literature dispute about whether the connectivity among the three sulfur atoms is a case of rapid tautomerization between two valence tautomers or a 3-center 4-electron bond.[2]

The reactions have been little studied. It forms a dinickel complex upon reaction with bis(allyl)nickel.[3]