

| |
| Names | |
|---|---|
| IUPAC name
zinc ethane-1,2-diylbis(dithiocarbamate) | |
| Other names
1,2 ethanediylbis[dithiocarbamodithioato](2−) zinc, | |
| Identifiers | |
3D model (JSmol) |
|
| 4165797 | |
| ChEBI | |
| ChemSpider |
|
| ECHA InfoCard | 100.031.970 |
| EC Number |
|
| KEGG |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2771 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H6N2S4Zn | |
| Molar mass | 275.8 g/mol (monomer) |
| Appearance | pale yellow powder |
| Hazards[1] | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
skin sensitizer |
| GHS labelling: | |

| |
| Warning | |
| H317, H335 | |
| P261, P271, P280, P302+P352, P304+P340, P312, P333+P313, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
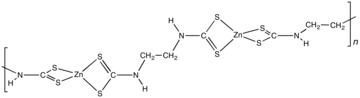
Zineb is the chemical compound with the formula {Zn[S2CN(H)CH2CH2N(H)CS2]}n. Structurally, it is classified as a coordination polymer and a dithiocarbamate complex. This pale yellow solid is used as fungicide.[2]
It is produced by treating ethylene bis(dithiocarbamate) sodium salt, "nabam", with zinc sulfate. This procedure can be carried out by mixing nabam and zinc sulfate in a spray tank.[3] Its uses include control of downy mildews, rusts, and redfire disease.[2] In the US it was once registered as a "General Use Pesticide", however all registrations were voluntarily cancelled following an EPA special review.[3] It continues to be used in many other countries.
Zineb is a polymeric complexofzinc with a dithiocarbamate.[2] The polymer is composed of Zn(dithiocarbamate)2 subunits linked by an ethylene (-CH2CH2-) backbone.[4] A reference compound is [Zn(S2CNEt2)2]2, which features a pair of tetrahedral Zn centers bridged by one sulfur center.[5]