出典: フリー百科事典『ウィキペディア(Wikipedia)』
GCK PDBに登録されている構造 PDB オルソログ検索: RCSB PDBe PDBj PDBのIDコード一覧 1V4S , 1V4T , 3A0I , 3F9M , 3FGU , 3FR0 , 3GOI , 3H1V , 3ID8 , 3IDH , 3QIC , 3S41 , 3VEV , 3VEY , 3VF6 , 4DCH , 4DHY , 4ISE , 4ISF , 4ISG , 4IWV , 4IXC , 4L3Q , 4LC9 , 3IMX , 4MLE , 4MLH , 4NO7 , 4RCH
識別子 記号 GCK 外部ID OMIM: 138079 MGI: 1270854 HomoloGene: 55440 GeneCards: GCK オルソログ 種 ヒト マウス Entrez Ensembl UniProt RefSeq RefSeq 場所 n/a Chr : 5.85 – 5.9 Mb PubMed 検索[2] [3] ウィキデータ
グ ル コ キ ナ ー ゼ ︵ 英 : g l u c o k i n a s e 、 EC 2 . 7 . 1 . 2 ︶ は 、 グ ル コ ー ス か ら グ ル コ ー ス - 6 - リ ン 酸 へ の リ ン 酸 化 を 促 進 す る 酵 素 で あ る 。 ヒ ト や 他 の 脊 椎 動 物 の 大 部 分 で は 、 グ ル コ キ ナ ー ゼ は 肝 臓 と 膵 臓 の 細 胞 で 発 現 し て い る 。 各 器 官 に お い て グ ル コ ー ス の セ ン サ ー と し て 機 能 す る こ と で 炭 水 化 物 の 代 謝 調 節 に 重 要 な 役 割 を 果 た し 、 食 事 後 や 絶 食 時 な ど の グ ル コ ー ス レ ベ ル の 上 昇 や 低 下 に 応 答 し て 代 謝 や 細 胞 機 能 の 変 化 を 開 始 さ せ る 。 こ の 酵 素 の 遺 伝 子 の 変 異 は 、 一 般 的 で な い 病 態 の 糖 尿 病 や 低 血 糖 症 を 引 き 起 こ す 。
グ ル コ キ ナ ー ゼ は ヘ キ ソ キ ナ ー ゼ の ア イ ソ ザ イ ム で あ り 、 他 の 3 つ の ヘ キ ソ キ ナ ー ゼ と 相 同 性 を 示 す [ 4 ] 。 ヘ キ ソ キ ナ ー ゼ は グ ル コ ー ス か ら グ ル コ ー ス - 6 - リ ン 酸 へ の リ ン 酸 化 を 媒 介 し 、 こ れ は グ リ コ ー ゲ ン 合 成 と 解 糖 系 の 双 方 の 第 一 段 階 で あ る 。 グ ル コ キ ナ ー ゼ の グ ル コ ー ス に 対 す る 親 和 性 は 他 の ヘ キ ソ キ ナ ー ゼ よ り も 低 い 。 他 の 3 つ の ヘ キ ソ キ ナ ー ゼ は ほ と ん ど の 組 織 や 器 官 で 解 糖 系 や グ リ コ ー ゲ ン 合 成 に 重 要 な 役 割 を 果 た す の に 対 し 、 グ ル コ キ ナ ー ゼ の 活 性 は い く つ か の 細 胞 種 に 限 ら れ て い る 。 こ の 低 い 親 和 性 の た め 、 生 理 的 条 件 下 に お け る グ ル コ キ ナ ー ゼ の 活 性 は グ ル コ ー ス 濃 度 に よ っ て 大 き く 変 動 す る [ 5 ] 。
こ の 酵 素 の 別 名 と し て は 、 h e x o k i n a s e IV 、 h e x o k i n a s e D 、 A T P : D - h e x o s e 6 - p h o s p h o t r a n s f e r a s e な ど が あ る 。 一 般 名 で あ る グ ル コ キ ナ ー ゼ は 、 生 理 的 条 件 下 で は グ ル コ ー ス に 対 し 特 異 性 を 示 す こ と に 由 来 す る 。
﹁ グ ル コ キ ナ ー ゼ ﹂ と い う 名 称 は ミ ス リ ー デ ィ ン グ で あ り 廃 止 す べ き で あ る と い う 主 張 も 一 部 に は 存 在 す る 。 そ れ は 、 こ の 酵 素 が 適 切 な 条 件 下 で は 他 の ヘ キ ソ ー ス に 対 し て も リ ン 酸 化 を 行 う た め 、 ま た 、 細 菌 に は グ ル コ ー ス に 対 し て 厳 密 な 特 異 性 を 示 し 、 こ の 酵 素 と は 遠 い 関 係 に あ る 酵 素 が 存 在 す る た め で あ る 。 ﹁ グ ル コ キ ナ ー ゼ ﹂ と い う 名 称 と EC 2 . 7 . 1 . 2 は 、 こ ち ら の 酵 素 に 対 し て 用 い ら れ る べ き で あ る 、 と の 主 張 が な さ れ て い る [ 5 ] [ 6 ] 。 し か し な が ら 、 医 学 や 哺 乳 類 の 生 理 学 に お い て は 依 然 と し て ﹁ グ ル コ キ ナ ー ゼ ﹂ と い う 名 称 が 用 い ら れ て い る 。
2 0 0 4 年 に は 、 哺 乳 類 で 新 た な グ ル コ ー ス に 対 す る キ ナ ー ゼ ︵ A D P 依 存 性 グ ル コ キ ナ ー ゼ ︵ 英 語 版 ︶ ︶ が 発 見 さ れ た [ 7 ] 。 こ の 遺 伝 子 は 、 こ の グ ル コ キ ナ ー ゼ と は 異 な り 原 始 的 生 物 の も の と 類 似 し て い る 。 こ の 酵 素 は A T P よ り も A D P に 依 存 し 、 低 酸 素 条 件 下 で よ り 効 率 的 に 機 能 す る 可 能 性 が 示 唆 さ れ て い る が 、 代 謝 に お け る 役 割 や 重 要 性 は 未 解 明 で あ る 。
基 質 と 産 物 [ 編 集 ] グ ル コ キ ナ ー ゼ の 生 理 的 に 重 要 な 基 質 は グ ル コ ー ス で あ り 、 最 も 重 要 な 産 物 は グ ル コ ー ス - 6 - リ ン 酸 で あ る 。 他 に 必 要 な 基 質 と し て は リ ン 酸 の 供 給 源 と な る ア デ ノ シ ン 三 リ ン 酸 ︵ A T P ︶ が あ り 、 リ ン 酸 が 除 去 さ れ て ア デ ノ シ ン 二 リ ン 酸 ︵ A D P ︶ に 変 換 さ れ る 。 グ ル コ キ ナ ー ゼ は 次 の 反 応 を 触 媒 す る 。
A T P は マ グ ネ シ ウ ム ︵ Mg ︶ を 補 因 子 と し て 結 合 し た 複 合 体 と し て 反 応 に 参 加 す る 。 さ ら に 特 定 の 条 件 下 で は 、 グ ル コ キ ナ ー ゼ は 他 の ヘ キ ソ キ ナ ー ゼ と 同 様 、 他 の ヘ キ ソ ー ス や 類 似 物 質 の リ ン 酸 化 を 誘 導 す る こ と が で き る 。 そ の た め よ り 正 確 に は 、 グ ル コ キ ナ ー ゼ が 触 媒 す る 一 般 的 反 応 は 次 の よ う に 記 述 さ れ る [ 6 ] 。
H e x o s e + M g A T P 2 − → H e x o s e - PO 2 − 3 + M g A D P − + H + 基 質 と な り う る ヘ キ ソ ー ス に は マ ン ノ ー ス 、 フ ル ク ト ー ス 、 グ ル コ サ ミ ン な ど が あ る が 、 こ れ ら の ヘ キ ソ ー ス に 対 す る 親 和 性 は 低 く 、 十 分 な 活 性 を 示 す た め に は 細 胞 内 で は み ら れ な い ほ ど の 高 濃 度 の 基 質 を 必 要 と す る [ 8 ] 。
速 度 論 [ 編 集 ] 2 つ の 重 要 な 速 度 論 的 性 質 に よ っ て 、 グ ル コ キ ナ ー ゼ は 他 の ヘ キ ソ キ ナ ー ゼ と 区 別 さ れ 、 グ ル コ ー ス の セ ン サ ー と し て の 特 別 な 機 能 が 可 能 と な っ て い る 。
(一) グ ル コ キ ナ ー ゼ は 他 の ヘ キ ソ キ ナ ー ゼ よ り も グ ル コ ー ス に 対 す る 親 和 性 が 低 い 。 グ ル コ キ ナ ー ゼ は 、 生 理 学 的 に 重 要 な 4 – 1 0 m M ︵ 7 2 – 1 8 0 m g / d L ︶ の 範 囲 で 、 グ ル コ ー ス 濃 度 の 上 昇 と と も に コ ン フ ォ メ ー シ ョ ン ま た は 機 能 の 変 化 が 生 じ る 。 約 8 mM ︵ 1 4 4 m g / d L ︶ の グ ル コ ー ス 濃 度 で 半 飽 和 と な る [ 9 ] [ 1 0 ] 。 (二) グ ル コ キ ナ ー ゼ は 反 応 産 物 で あ る グ ル コ ー ス - 6 - リ ン 酸 に よ っ て 阻 害 さ れ な い [ 9 ] 。 こ の 性 質 の た め 、 反 応 産 物 が 多 く 存 在 す る 条 件 下 で も シ グ ナ ル の 出 力 ︵ イ ン ス リ ン 放 出 の 誘 導 な ど ︶ を 継 続 す る こ と が で き る [ 1 0 ] 。 こ れ ら の 2 つ の 特 徴 に よ っ て 、 基 質 の 供 給 量 に よ っ て 代 謝 経 路 を 調 節 す る こ と が 可 能 と な る 。 す な わ ち 、 最 終 産 物 の 要 求 量 で は な く 、 グ ル コ ー ス の 供 給 量 に よ っ て グ ル コ キ ナ ー ゼ の 酵 素 反 応 の 速 度 は 決 定 さ れ る 。
グ ル コ キ ナ ー ゼ の 他 の 特 徴 と し て は 緩 や か な 協 同 性 が 挙 げ ら れ 、 ヒ ル 係 数 ︵ n H ︶ は 約 1 . 7 で あ る [ 1 0 ] 。 グ ル コ キ ナ ー ゼ に は グ ル コ ー ス の 結 合 部 位 が 1 つ し か 存 在 せ ず 、 基 質 協 同 性 を 示 す 唯 一 の 単 量 体 酵 素 で あ る 。 協 同 性 は 、 異 な る 反 応 速 度 を 持 つ 2 つ の 酵 素 状 態 間 の ﹁ 緩 や か な 転 移 ﹂ を 伴 う 過 程 に よ る も の で あ る と 想 定 さ れ て い る 。 優 勢 な 酵 素 状 態 が グ ル コ ー ス 濃 度 に 依 存 し て 変 化 す る 場 合 に は 、 観 察 さ れ て い る よ う な 見 か け 上 の 協 同 性 が 作 り 出 さ れ る [ 1 1 ] 。
こ の 協 同 性 の た め 、 グ ル コ キ ナ ー ゼ の グ ル コ ー ス と の 速 度 論 的 相 互 作 用 は 典 型 的 な ミ カ エ リ ス ・ メ ン テ ン 型 の 速 度 論 に は 従 わ な い 。 そ の た め 、 グ ル コ ー ス に 対 す る K m 値 よ り も 、 酵 素 の 5 0 % が 飽 和 し て 活 性 状 態 と な る 濃 度 で あ る 半 飽 和 濃 度 S 0 . 5 を 記 述 す る 方 が 正 確 で あ る 。
グ ル コ ー ス 濃 度 の 関 数 と し て 酵 素 活 性 を 記 述 し た 際 、 そ の 曲 線 の ﹁ 変 曲 点 ﹂ の 濃 度 は n H を 1 . 7 と す る と 約 4 m M で あ る [ 1 2 ] 。 言 い 換 え れ ば 、 グ ル コ ー ス 濃 度 の 生 理 的 正 常 範 囲 の 下 限 付 近 で あ る 約 7 2 m g / d L の 濃 度 に お い て 、 グ ル コ キ ナ ー ゼ の 活 性 は グ ル コ ー ス 濃 度 の 小 さ な 変 動 に 対 し 最 も 感 度 が 高 く な る 。
も う 一 方 の 基 質 で あ る M g - A T P と の 速 度 論 的 関 係 は 典 型 的 な ミ カ エ リ ス ・ メ ン テ ン 式 に よ っ て 記 述 さ れ 、 親 和 性 は 約 0 . 3 – 0 . 4 mM で 一 般 的 な 細 胞 内 A T P 濃 度 2 . 5 mM よ り も 十 分 に 低 い 。 ほ ぼ 常 に 過 剰 な A T P が 存 在 し て い る こ と は 、 A T P 濃 度 が グ ル コ キ ナ ー ゼ の 活 性 に 影 響 を 与 え る こ と は め っ た に な い こ と を 意 味 し て い る 。
双 方 の 基 質 が 飽 和 し て い る と き の グ ル コ キ ナ ー ゼ の 最 大 比 活 性 値 ま た は 回 転 数 ︵ k c a t ︶ は 6 2 s − 1 で あ る [ 9 ] 。 ヒ ト の グ ル コ キ ナ ー ゼ の 至 適 pH は 最 近 に な っ て 特 定 さ れ 、 p H 8 . 5 - 8 . 7 と 驚 く ほ ど 高 い こ と が 示 さ れ た [ 1 3 ] 。
グ ル コ ー ス の 結 合 部 位 は 、 複 数 の シ ス テ イ ン 残 基 の ス ル フ ヒ ド リ ル 基 に 囲 ま れ て い る 。 こ れ ら の シ ス テ イ ン 残 基 は C y s 2 3 0 を 除 い て 触 媒 過 程 に 必 須 で あ り 、 酸 化 に 伴 っ て 複 数 の 分 子 内 ジ ス ル フ ィ ド 結 合 が 形 成 さ れ て グ ル コ キ ナ ー ゼ は 不 安 定 化 さ れ る [ 1 4 ] 。 膵 臓 β 細 胞 で は 、 活 性 型 グ ル コ キ ナ ー ゼ と 不 活 性 型 と の 比 は 、 少 な く と も 部 分 的 に は 、 ス ル フ ヒ ド リ ル 基 の 酸 化 と ジ ス ル フ ィ ド 結 合 の 還 元 と の バ ラ ン ス に よ っ て 決 定 さ れ て い る 。
ヒ ト の グ ル コ キ ナ ー ゼ は 4 6 5 ア ミ ノ 酸 か ら な る 単 量 体 タ ン パ ク 質 で 、 分 子 量 は 約 5 0 , 0 0 0 で あ る 。 グ ル コ キ ナ ー ゼ の 立 体 構 造 に は 少 な く と も 2 つ の 割 れ 目 ︵ c l e f t ︶ が 存 在 し 、 一 方 は グ ル コ ー ス と M g - A T P が 結 合 す る 活 性 部 位 で 、 他 方 は 未 同 定 の ア ロ ス テ リ ッ ク 活 性 化 因 子 の 結 合 部 位 で あ る と 推 定 さ れ て い る [ 1 6 ] [ 1 7 ] 。
グ ル コ キ ナ ー ゼ の A T P 結 合 ド メ イ ン の 構 造 は 、 ヘ キ ソ キ ナ ー ゼ や 他 の タ ン パ ク 質 と 共 通 し て お り 、 そ の 共 通 構 造 は ア ク チ ン フ ォ ー ル ド と 名 付 け ら れ て い る [ 1 8 ] 。
遺 伝 学 [ 編 集 ] ヒ ト の グ ル コ キ ナ ー ゼ は 、 7 番 染 色 体 ︵ 英 語 版 ︶ の G C K 遺 伝 子 に コ ー ド さ れ て い る 。 こ の 遺 伝 子 は 10 個 の エ ク ソ ン か ら な る [ 1 9 ] [ 2 0 ] 。 他 の 動 物 の グ ル コ キ ナ ー ゼ の 遺 伝 子 は ヒ ト の G C K と 相 同 で あ る [ 9 ] [ 2 1 ] 。
こ の 遺 伝 子 の 特 徴 は 、 2 つ の プ ロ モ ー タ ー 領 域 か ら 始 ま る 点 で あ る [ 2 2 ] 。 5 ' 末 端 の 最 初 の エ ク ソ ン に は 、 組 織 特 異 的 な 2 つ の プ ロ モ ー タ ー 領 域 が 含 ま れ て い る 。 転 写 は ︵ 組 織 に よ っ て ︶ ど ち ら か の プ ロ モ ー タ ー か ら 開 始 さ れ 、 そ の 結 果 肝 臓 と 他 の 組 織 で は わ ず か に 異 な る 分 子 が 産 生 さ れ る 。 こ の グ ル コ キ ナ ー ゼ の 2 つ の ア イ ソ フ ォ ー ム は 分 子 の N 末 端 の 1 3 – 1 5 ア ミ ノ 酸 が 異 な る だ け で あ り 、 そ の 構 造 に は わ ず か な 差 異 し か 存 在 し な い 。 2 つ の ア イ ソ フ ォ ー ム は 同 じ 速 度 論 的 ・ 機 能 的 性 質 を 有 す る [ 5 ] 。
5 ' 末 端 に あ る 1 番 目 の プ ロ モ ー タ ー ︵ 上 流 プ ロ モ ー タ ー ま た は 神 経 内 分 泌 プ ロ モ ー タ ー と 呼 ば れ る ︶ は 膵 島 細 胞 、 神 経 組 織 、 小 腸 の 上 皮 細 胞 ︵ エ ン テ ロ サ イ ト ︶ で 活 性 が あ り 、 グ ル コ キ ナ ー ゼ の ﹁ 神 経 内 分 泌 型 ア イ ソ フ ォ ー ム ﹂ を 産 生 す る [ 2 2 ] 。 2 番 目 の プ ロ モ ー タ ー ︵ 下 流 プ ロ モ ー タ ー ま た は 肝 臓 プ ロ モ ー タ ー と 呼 ば れ る ︶ は 肝 細 胞 で 活 性 が あ り 、 ﹁ 肝 臓 型 ア イ ソ フ ォ ー ム ﹂ の 産 生 を 指 示 す る [ 2 3 ] 。 2 つ の プ ロ モ ー タ ー に は ほ と ん ど ま た は 全 く 配 列 相 同 性 が な く 、 3 0 k bp の 配 列 で 隔 て ら れ て い る [ 5 ] 。 ア イ ソ フ ォ ー ム 間 の 機 能 的 差 異 は 、 こ れ ま で 示 さ れ て い な い 。 こ の 2 つ の プ ロ モ ー タ ー は 排 他 的 に 機 能 し 、 異 な る 調 節 因 子 の セ ッ ト に よ っ て 制 御 さ れ て い る た め 、 グ ル コ キ ナ ー ゼ の 発 現 は 組 織 の タ イ プ に よ っ て 別 々 の 調 節 を 受 け て い る [ 5 ] 。 2 つ の プ ロ モ ー タ ー は 、 大 き く 2 つ の 機 能 カ テ ゴ リ に 対 応 す る 。 肝 臓 で は 、 グ ル コ キ ナ ー ゼ は 利 用 可 能 な グ ル コ ー ス の 大 量 処 理 の 出 発 点 と し て 機 能 す る 。 一 方 、 神 経 内 分 泌 細 胞 で は 、 全 身 の 炭 水 化 物 代 謝 に 影 響 を 与 え る 細 胞 応 答 を 誘 導 す る セ ン サ ー と し て 機 能 す る 。
組 織 分 布 [ 編 集 ] グ ル ク コ キ ナ ー ゼ は 哺 乳 類 の 4 種 類 の 組 織 ︵ 肝 臓 、 膵 臓 、 小 腸 、 脳 ︶ の 特 定 の 細 胞 に 存 在 し て い る 。 こ れ ら 全 て が 血 糖 値 の 上 昇 や 低 下 に 対 す る 応 答 に 重 要 な 役 割 を 果 た す 。
● グ ル コ キ ナ ー ゼ は 肝 臓 の 主 要 な 細 胞 で あ る 肝 細 胞 に 存 在 し て い る 。 炭 水 化 物 を 含 む 食 事 の 消 化 に よ っ て 血 糖 が 増 加 し 、 イ ン ス リ ン レ ベ ル が 上 昇 し た と き に は 、 肝 細 胞 は 血 中 か ら グ ル コ ー ス を 除 去 し 、 グ リ コ ー ゲ ン の 形 で 貯 蔵 す る 。 消 化 と 吸 収 が 完 了 し た 後 に は 、 肝 臓 は グ ル コ ー ス 以 外 の 基 質 や グ リ コ ー ゲ ン か ら グ ル コ ー ス を 産 生 し て ︵ こ の 過 程 は そ れ ぞ れ 糖 新 生 、 グ リ コ ー ゲ ン 分 解 と 呼 ば れ る ︶ 血 中 へ 放 出 し 、 絶 食 時 も 血 糖 値 を 適 切 に 維 持 す る 。 グ ル コ キ ナ ー ゼ の 活 性 は グ ル コ ー ス 濃 度 が 上 昇 す る と 迅 速 に 増 大 し 、 給 餌 状 態 と 絶 食 状 態 の 間 で 肝 臓 の 炭 水 化 物 代 謝 を シ フ ト さ せ る 中 心 的 な 代 謝 ス イ ッ チ と し て 機 能 す る [ 2 4 ] [ 2 5 ] 。 グ ル コ キ ナ ー ゼ に よ る グ ル コ ー ス か ら グ ル コ ー ス - 6 - リ ン 酸 へ の リ ン 酸 化 は 、 グ ル コ ー ス の グ リ コ ー ゲ ン と し て の 貯 蔵 や 解 糖 系 に よ る 蓄 積 を 促 進 す る 。 肝 臓 プ ロ モ ー タ ー に よ っ て 、 肝 臓 で は 神 経 内 分 泌 細 胞 と は 異 な る 調 節 が 可 能 と な っ て い る [ 2 6 ] [ 2 7 ] 。 ● 膵 臓 、 腸 、 脳 の 神 経 内 分 泌 細 胞 は 、 グ ル コ キ ナ ー ゼ の 合 成 、 調 節 、 機 能 に 関 し て い く つ か の 共 通 し た 側 面 が 存 在 す る [ 2 8 ] 。 こ れ ら の 組 織 を こ こ で は ま と め て ﹁ 神 経 内 分 泌 ﹂ 細 胞 と 呼 ぶ 。
● 膵 島 の β 細 胞 と α 細 胞 ● β 細 胞 は グ ル コ ー ス レ ベ ル の 上 昇 に 応 答 し て イ ン ス リ ン を 放 出 す る 。 イ ン ス リ ン は 多 く の 細 胞 種 で グ ル コ ー ス の 取 り 込 み と 利 用 を 可 能 に し 、 肝 臓 へ グ リ コ ー ゲ ン 合 成 の シ グ ナ ル を 伝 達 す る 。 α 細 胞 は グ ル コ ー ス レ ベ ル の 上 昇 に 応 答 し て グ ル カ ゴ ン の 分 泌 を 低 下 さ せ 、 血 糖 値 が 低 い と き に は よ り 多 く の グ ル カ ゴ ン を 分 泌 す る 。 グ ル カ ゴ ン は 肝 臓 で グ リ コ ー ゲ ン 分 解 と 血 中 へ の グ ル コ ー ス の 放 出 の シ グ ナ ル と し て 機 能 す る 。 膵 臓 の β 細 胞 で は 、 グ ル コ キ ナ ー ゼ は 重 要 な 調 節 酵 素 で あ る 。 グ ル コ キ ナ ー ゼ は グ ル コ ー ス の セ ン サ ー と し て 機 能 し 、 血 糖 値 に 応 じ て イ ン ス リ ン の 分 泌 を 調 節 す る 。 グ ル コ キ ナ ー ゼ を コ ー ド す る 遺 伝 子 の 変 異 は 、 高 血 糖 症 と 低 血 糖 症 の 双 方 を 引 き 起 こ す 可 能 性 が あ る [ 2 9 ] 。 ● 視 床 下 部 の グ ル コ ー ス 感 受 性 ニ ュ ー ロ ン ● グ ル コ ー ス レ ベ ル の 上 昇 と 低 下 に 応 答 し て 、 視 床 下 部 の 細 胞 は 分 極 ま た は 脱 分 極 を 行 う 。 低 血 糖 に 対 す る 中 枢 神 経 系 の 応 答 は 自 律 神 経 系 を 介 し て 膵 臓 の α 細 胞 へ 伝 達 さ れ 、 グ ル カ ゴ ン の 分 泌 を 促 進 す る 。 グ ル コ キ ナ ー ゼ は こ こ で も 同 様 に グ ル コ ー ス シ グ ナ ル 伝 達 に 関 与 し て い る と 考 え ら れ る [ 3 0 ] 。 グ ル コ キ ナ ー ゼ は 脳 下 垂 体 前 葉 の 細 胞 に も 見 つ か る [ 3 1 ] 。 ● 小 腸 の エ ン テ ロ サ イ ト
● グ ル コ キ ナ ー ゼ の セ ン サ ー シ ス テ ム の う ち 最 も 理 解 が 進 ん で い な い 。 β 細 胞 と 同 様 に こ れ ら の 細 胞 で も グ ル コ キ ナ ー ゼ が 消 化 中 の グ ル コ ー ス 流 入 に 対 す る セ ン サ ー と し て 機 能 し 、 イ ン ク レ チ ン に よ る 食 事 中 の イ ン ス リ ン 分 泌 の 増 幅 な ど へ 関 与 す る こ と が 推 測 さ れ て い た が 、 主 要 な 役 割 を 果 た し て い な い と の 報 告 も あ る [ 2 8 ] [ 3 2 ] 。 種 間 分 布 [ 編 集 ] 肝 臓 型 グ ル コ キ ナ ー ゼ は 脊 椎 動 物 に 広 く 存 在 し て い る が 、 普 遍 的 に 存 在 し て い る わ け で は な い 。 遺 伝 子 構 造 や ア ミ ノ 酸 配 列 は ほ と ん ど の 哺 乳 類 で 高 度 に 保 存 さ れ て い る 。 し か し い く つ か の 例 外 も 存 在 す る 。 一 部 の 爬 虫 類 、 鳥 類 、 両 生 類 や 魚 類 も グ ル コ キ ナ ー ゼ を 有 す る の に 対 し 、 ネ コ と コ ウ モ リ で は 未 発 見 で あ る [ 3 3 ] 。 グ ル コ キ ナ ー ゼ が 膵 臓 や 他 の 器 官 で も 同 様 に 存 在 し て い る の か に つ い て は 未 解 明 で あ る 。 肝 臓 に お け る グ ル コ キ ナ ー ゼ の 存 在 は 、 動 物 の 食 事 に 含 ま れ る 炭 水 化 物 の 量 や グ ル コ ー ス 除 去 の 必 要 性 を 反 映 し て い る と 推 測 さ れ て い る [ 3 4 ] 。
機 能 と 調 節 [ 編 集 ] 哺 乳 類 で は グ ル コ キ ナ ー ゼ の 大 部 分 は 肝 臓 に 存 在 し 、 グ ル コ キ ナ ー ゼ は 肝 細 胞 に お け る ヘ キ ソ キ ナ ー ゼ 活 性 の 約 9 5 % を 占 め る [ 3 5 ] 。 グ ル コ キ ナ ー ゼ に よ る グ ル コ ー ス の グ ル コ ー ス - 6 - リ ン 酸 へ の リ ン 酸 化 は 、 肝 臓 に お け る グ リ コ ー ゲ ン 合 成 と 解 糖 系 の 第 一 段 階 で あ る 。
十 分 な グ ル コ ー ス の 存 在 下 で は 、 グ リ コ ー ゲ ン 合 成 は 肝 細 胞 の 周 縁 部 で 進 行 す る [ 3 6 ] 。 グ ル コ キ ナ ー ゼ に よ る 反 応 産 物 で あ る グ ル コ ー ス - 6 - リ ン 酸 は グ リ コ ー ゲ ン 合 成 の 主 要 な 基 質 で あ り 、 グ ル コ キ ナ ー ゼ は グ リ コ ー ゲ ン 合 成 と 機 能 的 ・ 調 節 的 に 密 接 に 関 係 し て い る 。 最 も 活 性 が 高 い 時 に は 、 グ ル コ キ ナ ー ゼ と グ リ コ ー ゲ ン シ ン タ ー ゼ は 、 グ リ コ ー ゲ ン 合 成 が 行 わ れ る 細 胞 質 周 縁 部 の 同 じ 領 域 に 位 置 し て い る よ う で あ る [ 3 7 ] 。 グ ル コ ー ス - 6 - リ ン 酸 の 供 給 は グ リ コ ー ゲ ン 合 成 の 主 要 基 質 と し て 合 成 速 度 に 影 響 を 与 え る だ け で な く 、 直 接 的 な グ リ コ ー ゲ ン シ ン タ ー ゼ の 活 性 促 進 や 、 グ リ コ ー ゲ ン ホ ス ホ リ ラ ー ゼ の 阻 害 を 行 う [ 3 8 ] 。
グ ル コ キ ナ ー ゼ の 活 性 は 、 摂 食 や 絶 食 な ど の グ ル コ ー ス 供 給 の 変 化 に 応 答 し て 迅 速 な 増 大 と 低 下 が 起 こ る 。 調 節 は い く つ か の レ ベ ル で 行 わ れ 、 多 く の 因 子 の 影 響 を 受 け る が 、 主 に 2 つ の 機 構 が 影 響 を 受 け る 。
(一) グ ル コ キ ナ ー ゼ の 活 性 は グ ル コ キ ナ ー ゼ 調 節 タ ン パ ク 質 ︵ G K R P ︶ の 作 用 に よ っ て 増 減 す る 。 こ の タ ン パ ク 質 の 作 用 は グ ル コ ー ス や フ ル ク ト ー ス と い っ た 低 分 子 の 影 響 を 受 け る [ 2 4 ] 。 (二) グ ル コ キ ナ ー ゼ の 量 は 新 た な タ ン パ ク 質 の 合 成 に よ っ て 増 加 す る 。 イ ン ス リ ン は 転 写 の 増 加 の 主 要 な シ グ ナ ル で あ り 、 主 に ス テ ロ ー ル 調 節 エ レ メ ン ト 結 合 タ ン パ ク 質 ︵ 英 語 版 ︶ 1 c ︵ S R E B P - 1 c ︶ と 呼 ば れ る 転 写 因 子 を 介 し て 行 わ れ る [ 2 5 ] 。 S R E B P - 1 c を 介 し た イ ン ス リ ン の 作 用 は 、 肝 細 胞 に お け る グ ル コ キ ナ ー ゼ 遺 伝 子 の 転 写 を 直 接 活 性 化 す る 最 も 重 要 な 因 子 で あ る と 考 え ら れ て い る 。 S R E B P - 1 c は 塩 基 性 ヘ リ ッ ク ス ル ー プ ヘ リ ッ ク ス ・ ロ イ シ ン ジ ッ パ ー 型 の 転 写 活 性 化 因 子 で あ る 。 グ ル コ キ ナ ー ゼ 遺 伝 子 の 最 初 の エ ク ソ ン の 肝 臓 型 プ ロ モ ー タ ー に は ス テ ロ ー ル 応 答 エ レ メ ン ト ま た は E - b o x が 存 在 し 、 こ れ ら が 肝 細 胞 に お け る 主 要 な イ ン ス リ ン 応 答 エ レ メ ン ト と し て 機 能 し て い る よ う で あ る [ 2 6 ] [ 2 7 ] 。 S R E B P - 1 c は 肝 細 胞 で の グ ル コ キ ナ ー ゼ の 転 写 の た め に 不 可 欠 で あ る と 考 え ら れ て き た が 、 S R E B P - 1 c ノ ッ ク ア ウ ト マ ウ ス で も 高 炭 水 化 物 食 に 応 答 し た グ ル コ キ ナ ー ゼ の 転 写 が 正 常 に 行 わ れ る と い う 報 告 も 存 在 す る [ 3 9 ] 。
フ ル ク ト ー ス - 2 , 6 - ビ ス リ ン 酸 ︵ F 2 , 6 P 2 ︶ も グ ル コ キ ナ ー ゼ の 転 写 を 促 進 す る が 、 こ の 促 進 は S R E B P - 1 c よ り は A k t を 介 し て 行 わ れ て い る よ う で あ る 、 こ の 作 用 が イ ン ス リ ン 受 容 体 活 性 化 の 下 流 の 影 響 の 1 つ で あ る の か 、 そ れ と も イ ン ス リ ン の 作 用 に 依 存 し な い も の で あ る の か に つ い て は 明 ら か で は な い [ 4 0 ] [ 4 1 ] 。
肝 細 胞 で の 転 写 調 節 に 関 与 し て い る 可 能 性 が あ る 他 の 因 子 と し て は 次 の よ う な も の が あ る [ 3 3 ] 。
(一) H N F 4 α ︵ H e p a t i c n u c l e a r f a c t o r 4 - a l p h a ︶ は 、 炭 水 化 物 や 脂 質 の 代 謝 に 関 わ る 多 く の 酵 素 の 遺 伝 子 の 転 写 に 重 要 な 核 内 受 容 体 で あ り 、 グ ル コ キ ナ ー ゼ 遺 伝 子 の 転 写 も 活 性 化 す る 。 (二) U S F 1 ︵ 英 語 版 ︶ ︵ U p s t r e a m s t i m u l a t o r y f a c t o r 1 ︶ は 、 他 の 塩 基 性 ヘ リ ッ ク ス ル ー プ ヘ リ ッ ク ス ・ ロ イ シ ン ジ ッ パ ー 型 転 写 活 性 化 因 子 で あ る 。 (三) H N F 6 ︵ 英 語 版 ︶ ︵ H e p a t i c n u c l e a r f a c t o r 6 ︶ は 、 o n e - c u t ク ラ ス に 分 類 さ れ る ホ メ オ ド メ イ ン 型 転 写 調 節 因 子 で あ る 。 H N F 6 は 、 グ ル コ ー ス - 6 - ホ ス フ ァ タ ー ゼ や ホ ス ホ エ ノ ー ル ピ ル ビ ン 酸 カ ル ボ キ シ キ ナ ー ゼ な ど の 糖 新 生 に 関 わ る 酵 素 の 転 写 も 調 節 す る 。 ホ ル モ ン と 食 事 [ 編 集 ] イ ン ス リ ン は 、 肝 臓 で の グ ル コ キ ナ ー ゼ の 発 現 と 活 性 に 直 接 的 ・ 間 接 的 な 影 響 を 与 え る 最 も 重 要 な ホ ル モ ン で あ る 。 イ ン ス リ ン は グ ル コ キ ナ ー ゼ の 転 写 と 活 性 の 双 方 に 対 し 、 複 数 の 直 接 的 ・ 間 接 的 経 路 を 介 し て 影 響 を 与 え て い る よ う で あ る 。 グ ル コ ー ス レ ベ ル の 上 昇 は グ ル コ キ ナ ー ゼ の 活 性 を 上 昇 さ せ る が 、 イ ン ス リ ン の 上 昇 は そ れ と は 独 立 し て グ ル コ キ ナ ー ゼ の 合 成 を 誘 導 し 、 そ の 影 響 を 増 幅 さ せ る [ 4 2 ] 。 グ ル コ キ ナ ー ゼ の 転 写 は イ ン ス リ ン レ ベ ル の 上 昇 後 1 時 間 以 内 に 上 昇 し 始 め る [ 4 3 ] 。
イ ン ス リ ン に よ る グ ル コ キ ナ ー ゼ の 誘 導 に は 、 イ ン ス リ ン が 作 用 す る 主 要 な 細 胞 内 経 路 で あ る E R K 1 / 2 カ ス ケ ー ド [ 4 4 ] と P I 3 K カ ス ケ ー ド [ 4 5 ] の 双 方 が 関 係 し て い る 。 後 者 は 転 写 因 子 F O X O 1 の 調 節 を 介 し て 行 わ れ て い る よ う で あ る [ 4 5 ] 。
グ ル カ ゴ ン は グ リ コ ー ゲ ン 合 成 に 対 し て イ ン ス リ ン に 拮 抗 す る 影 響 を 与 え る が 、 グ ル カ ゴ ン と そ の 細 胞 内 セ カ ン ド メ ッ セ ン ジ ャ ー で あ る c A M P は 、 イ ン ス リ ン 存 在 下 で も グ ル コ キ ナ ー ゼ の 転 写 を 活 性 を 抑 制 す る [ 4 2 ] 。
ト リ ヨ ー ド チ ロ ニ ン な ど 他 の ホ ル モ ン も 特 定 の 条 件 下 で グ ル コ キ ナ ー ゼ に 対 し 許 容 効 果 ま た は 促 進 効 果 を 示 す [ 4 6 ] 。 ビ オ チ ン と レ チ ノ イ ン 酸 も グ ル コ キ ナ ー ゼ の 転 写 と 活 性 を 上 昇 さ せ る [ 4 7 ] [ 4 8 ] 。 長 鎖 ア シ ル C o A は グ ル コ キ ナ ー ゼ の 活 性 を 阻 害 す る [ 4 9 ] 。
肝 細 胞 で は 、 グ ル コ キ ナ ー ゼ は グ ル コ キ ナ ー ゼ 調 節 タ ン パ ク 質 ︵ G K R P ︶ に よ っ て 迅 速 に 活 性 化 と 不 活 性 化 が 行 わ れ る 。 G K R P は グ ル コ キ ナ ー ゼ を 不 活 性 状 態 で 蓄 え て お く 機 能 を 持 ち 、 門 脈 の グ ル コ ー ス レ ベ ル の 上 昇 に 応 答 し て グ ル コ キ ナ ー ゼ は 迅 速 に 利 用 可 能 な 状 態 と な る [ 5 0 ] 。
G K R P は 肝 細 胞 の 核 と 細 胞 質 の 間 を 移 行 し 、 マ イ ク ロ フ ィ ラ メ ン ト の 細 胞 骨 格 に 結 合 し て い る 可 能 性 が あ る [ 3 3 ] 。 G K R P は グ ル コ キ ナ ー ゼ と 1 : 1 で 複 合 体 を 形 成 し 、 G K R P は グ ル コ ー ス の 競 合 的 阻 害 剤 と し て 機 能 す る [ 5 1 ] 。 グ ル コ ー ス と フ ル ク ト ー ス の レ ベ ル が 低 い と き に は 、 グ ル コ キ ナ ー ゼ - G K R P 複 合 体 は 核 へ 隔 離 さ れ て い る [ 5 2 ] 。 核 へ の 隔 離 は グ ル コ キ ナ ー ゼ を 細 胞 質 の プ ロ テ ア ー ゼ に よ る 分 解 か ら 保 護 す る 役 割 も 持 っ て い る 可 能 性 が あ る [ 5 3 ] 。 グ ル コ ー ス レ ベ ル の 上 昇 に 応 答 し て 、 グ ル コ キ ナ ー ゼ は 迅 速 に G K R P か ら 遊 離 す る [ 5 2 ] 。 β 細 胞 と は 異 な り 、 肝 細 胞 の グ ル コ キ ナ ー ゼ は ミ ト コ ン ド リ ア と は 結 合 し て い な い [ 5 4 ] 。
微 量 ︵ μ M 程 度 ︶ の フ ル ク ト ー ス は 、 ケ ト ヘ キ ソ キ ナ ー ゼ に よ る フ ル ク ト ー ス - 1 - リ ン 酸 ︵ F 1 P ︶ へ の リ ン 酸 化 の 後 、 グ ル コ キ ナ ー ゼ の G K R P か ら の 解 離 を 促 進 す る [ 5 1 ] 。 こ の 微 量 の フ ル ク ト ー ス に 対 す る 感 受 性 は 、 G K R P 、 グ ル コ キ ナ ー ゼ 、 そ し て ケ ト ヘ キ ソ キ ナ ー ゼ に よ る ﹁ フ ル ク ト ー ス 検 知 シ ス テ ム ﹂ を 可 能 に し て い る 。 こ の シ ス テ ム は 混 合 炭 水 化 物 を 含 む 食 事 が 消 化 さ れ て い る と い う シ グ ナ ル を 発 し 、 グ ル コ ー ス の 利 用 を 加 速 さ せ る 。 一 方 で 、 フ ル ク ト ー ス - 6 - リ ン 酸 ︵ F 6 P ︶ は G K R P に よ る GK の 結 合 を 強 化 す る [ 5 1 ] 。 F 6 P は 、 グ リ コ ー ゲ ン 分 解 や 糖 新 生 が 行 わ れ て い る 際 に 、 グ ル コ キ ナ ー ゼ に よ る グ ル コ ー ス の リ ン 酸 化 を 低 下 さ せ る 。 F 1 P と F 6 P は G K R P の 重 複 す る 部 位 に 結 合 す る [ 5 1 ] 。 F 1 P ま た は F 6 P の 結 合 に よ っ て G K R P は 2 つ の 異 な る コ ン フ ォ メ ー シ ョ ン を と り 、 一 方 は グ ル コ キ ナ ー ゼ に 結 合 で き る 構 造 、 他 方 は 結 合 で き な い 構 造 と な る と 考 え ら れ て い る 。
体 内 の グ ル コ キ ナ ー ゼ の 大 部 分 は 肝 臓 に 存 在 す る が 、 膵 臓 の α 細 胞 と β 細 胞 、 視 床 下 部 の 特 定 種 の ニ ュ ー ロ ン 、 腸 の 特 定 の 細 胞 ︵ エ ン テ ロ サ イ ト ︶ に 少 量 存 在 す る グ ル コ キ ナ ー ゼ が 炭 水 化 物 代 謝 の 調 節 に 重 要 な 役 割 を 果 た す こ と が 判 明 し て き て い る 。 こ れ ら の 細 胞 種 は ま と め て 神 経 内 分 泌 組 織 と 呼 ば れ 、 共 通 し た 神 経 内 分 泌 型 プ ロ モ ー タ ー か ら の 転 写 な ど 、 グ ル コ キ ナ ー ゼ の 調 節 と 機 能 に 関 し て 共 通 の 側 面 が 存 在 し て い る 。 こ れ ら の 神 経 内 分 泌 細 胞 の 中 で も 、 膵 島 の β 細 胞 が 最 も 研 究 さ れ て お り 、 理 解 が 進 ん で い る 。 β 細 胞 で 発 見 さ れ た 調 節 関 係 の 多 く は 、 他 の 神 経 内 分 泌 組 織 の グ ル コ キ ナ ー ゼ に も 存 在 し て い る 可 能 性 が 高 い 。
イ ン ス リ ン 放 出 の た め の シ グ ナ ル [ 編 集 ] 膵 島 の β 細 胞 で は 、 グ ル コ キ ナ ー ゼ の 活 性 は 血 糖 値 の 上 昇 に 応 答 し た イ ン ス リ ン 分 泌 の 主 要 な 制 御 を 行 っ て い る 。 解 糖 系 な ど の 細 胞 呼 吸 に よ っ て グ ル コ ー ス - 6 - リ ン 酸 が 消 費 さ れ て A T P 量 が 増 加 す る と 、 イ ン ス リ ン の 放 出 に 至 る 一 連 の 過 程 が 開 始 さ れ る 。 A T P 量 の 増 加 に よ っ て A T P 感 受 性 カ リ ウ ム チ ャ ネ ル が 閉 じ 、 細 胞 膜 の 脱 分 極 、 細 胞 内 の カ ル シ ウ ム レ ベ ル の 上 昇 、 イ ン ス リ ン 分 泌 顆 粒 の 膜 へ の 融 合 、 そ し て 血 中 へ の イ ン ス リ ン の 放 出 が 引 き 起 こ さ れ る 。 こ の 機 構 は グ ル コ ー ス に 応 答 し た イ ン ス リ ン 放 出 の 第 一 波 を 構 成 す る [ 5 5 ] 。
β 細 胞 に お け る 調 節 [ 編 集 ] グ ル コ ー ス は 上 述 の 協 同 的 効 果 に よ っ て グ ル コ キ ナ ー ゼ の 活 性 を 速 や か に 増 大 さ せ る 。
β 細 胞 に お け る グ ル コ キ ナ ー ゼ 活 性 の 調 節 に 重 要 な 2 つ 目 の 因 子 は 、 解 糖 系 の 調 節 に 関 与 す る ﹁ 二 機 能 酵 素 ﹂ ︵ " b i f u n c t i o n a l e n z y m e " 、 ホ ス ホ フ ル ク ト キ ナ ー ゼ 2 / フ ル ク ト ー ス - 2 , 6 - ビ ス ホ ス フ ァ タ ー ゼ ︶ と の 直 接 的 な タ ン パ ク 質 間 相 互 作 用 で あ る 。 こ の 物 理 的 な 結 合 は 、 グ ル コ キ ナ ー ゼ の 触 媒 に 適 し た コ ン フ ォ メ ー シ ョ ン を 安 定 化 し ︵ G K R P の 結 合 と ほ ぼ 反 対 の 作 用 で あ る ︶ 、 活 性 を 向 上 さ せ る [ 5 6 ] 。
早 け れ ば 15 分 以 内 に 、 グ ル コ ー ス に よ る イ ン ス リ ン を 介 し た G C K 遺 伝 子 の 転 写 と グ ル コ キ ナ ー ゼ の 合 成 の 促 進 が み ら れ る 。 イ ン ス リ ン は β 細 胞 で 産 生 さ れ 、 そ の 一 部 は β 細 胞 の B 型 ア イ ソ フ ォ ー ム の イ ン ス リ ン 受 容 体 に 作 用 し 、 自 己 分 泌 に よ っ て グ ル コ キ ナ ー ゼ の 活 性 を 増 幅 す る ポ ジ テ ィ ブ フ ィ ー ド バ ッ ク ル ー プ を 形 成 す る 。 さ ら に 、 イ ン ス リ ン は A 型 イ ソ フ ォ ー ム の 受 容 体 を 介 し て 自 身 の 転 写 を 促 進 し 、 さ ら な る 増 幅 が 行 わ れ る [ 5 7 ] 。
G C K 遺 伝 子 の 転 写 は 上 流 ま た は 神 経 内 分 泌 型 プ ロ モ ー タ ー か ら 開 始 さ れ る 。 肝 臓 型 プ ロ モ ー タ ー と 対 照 的 に 、 こ の プ ロ モ ー タ ー に は イ ン ス リ ン に よ っ て 誘 導 さ れ る 他 の 遺 伝 子 の プ ロ モ ー タ ー と 相 同 な エ レ メ ン ト が 存 在 す る 。 活 性 化 を 担 う 転 写 因 子 と し て は P d x - 1 や P P A R γ の 可 能 性 が あ る [ 5 8 ] [ 5 9 ] 。 P d x - 1 は 膵 臓 の 分 化 に 関 与 す る ホ メ オ ド メ イ ン 型 の 転 写 因 子 で あ る 。 P P A R γ は 、 グ リ タ ゾ ン に 応 答 し て イ ン ス リ ン 感 受 性 を 向 上 さ せ る 核 内 受 容 体 で あ る 。
イ ン ス リ ン 分 泌 顆 粒 と の 結 合 [ 編 集 ] β 細 胞 の 細 胞 質 の グ ル コ キ ナ ー ゼ は 、 全 て で は な い も の の 、 多 く が イ ン ス リ ン 分 泌 顆 粒 や ミ ト コ ン ド リ ア と 結 合 し て い る 。 こ の 結 合 の 割 合 は グ ル コ ー ス の 上 昇 と イ ン ス リ ン の 分 泌 に 応 答 し て 迅 速 に 低 下 す る 。 肝 臓 の G K R P と 同 様 に 、 こ の 結 合 は グ ル コ ー ス が 上 昇 時 に す ば や く 利 用 で き る よ う 、 グ ル コ キ ナ ー ゼ を 分 解 か ら 保 護 す る 役 割 が あ る と 示 唆 さ れ て い る 。 転 写 を 介 し た 過 程 よ り も す ば や く グ ル コ キ ナ ー ゼ の 活 性 を 増 大 さ せ る 効 果 が あ る [ 6 0 ] 。
α 細 胞 に お け る グ ル カ ゴ ン の 抑 制 [ 編 集 ] グ ル コ キ ナ ー ゼ は 膵 臓 の α 細 胞 で も グ ル コ ー ス の 検 知 を 行 う こ と が 提 唱 さ れ て い る 。 α 細 胞 は β 細 胞 や 他 の 細 胞 と 混 ざ っ た 状 態 で 膵 島 に 存 在 す る 。 β 細 胞 は グ ル コ ー ス レ ベ ル の 上 昇 に 対 し イ ン ス リ ン を 分 泌 す る こ と で 応 答 す る が 、 α 細 胞 は グ ル カ ゴ ン の 分 泌 を 低 下 さ せ る こ と で 応 答 す る 。 血 糖 値 が 低 血 糖 症 レ ベ ル に ま で 低 下 す る と 、 α 細 胞 は グ ル カ ゴ ン を 放 出 す る 。 グ ル カ ゴ ン は 肝 細 胞 に 対 す る イ ン ス リ ン の 作 用 を 遮 る タ ン パ ク 質 ホ ル モ ン で あ り 、 肝 細 胞 で の グ リ コ ー ゲ ン 分 解 、 糖 新 生 、 グ ル コ キ ナ ー ゼ 活 性 の 低 下 を 誘 導 す る 。 β 細 胞 に お け る グ ル コ キ ナ ー ゼ を 介 し た イ ン ス リ ン 応 答 ほ ど で は な い が 、 α 細 胞 で の グ ル コ キ ナ ー ゼ を 介 し た グ ル カ ゴ ン の 分 泌 抑 制 に 関 す る 証 拠 も 蓄 積 が 進 ん で お り 、 広 く 受 け 入 れ ら れ つ つ あ る [ 6 1 ] 。
臨 床 的 意 義 [ 編 集 ] イ ン ス リ ン は グ ル コ キ ナ ー ゼ の 合 成 の 調 節 因 子 の 1 つ で あ り 、 す べ て の タ イ プ の 糖 尿 病 に お い て グ ル コ キ ナ ー ゼ の 合 成 と 活 性 は さ ま ざ ま な 機 構 に よ っ て 低 下 し て い る 。 グ ル コ キ ナ ー ゼ の 活 性 は 、 特 に β 細 胞 に お い て 、 酸 化 ス ト レ ス に 対 し て 敏 感 で あ る 。
ヒ ト の グ ル コ キ ナ ー ゼ 遺 伝 子 G C K に は 多 数 の 変 異 が 同 定 さ れ て お り 、 そ れ ら は グ ル コ ー ス の 結 合 や リ ン 酸 化 の 効 率 を 変 化 さ せ る 。 そ の 結 果 、 β 細 胞 の グ ル コ ー ス 応 答 性 イ ン ス リ ン 分 泌 の 感 受 性 が 増 加 ま た は 低 下 し 、 臨 床 的 に 重 要 な 高 血 糖 ま た は 低 血 糖 状 態 と な る 。
糖 尿 病 [ 編 集 ] G C K 遺 伝 子 の 変 異 は グ ル コ キ ナ ー ゼ の 機 能 効 率 を 低 下 さ せ る 。 酵 素 活 性 が 低 下 し た ア レ ル の ヘ テ ロ 接 合 型 と な る こ と に よ っ て 、 イ ン ス リ ン 放 出 の 閾 値 が 上 昇 し 、 持 続 性 の 軽 症 高 血 糖 症 と な る 。 こ の 状 況 は 若 年 発 症 成 人 型 糖 尿 病 ︵ 英 語 版 ︶ 2 型 ︵ M O D Y 2 ︶ と 呼 ば れ る 。 最 近 の 報 告 で は 患 者 で は 7 9 1 種 類 の G C K の 変 異 が 観 察 さ れ て お り 、 そ の う ち 4 8 9 は M O D Y を 引 き 起 こ し 、 そ の た め グ ル コ キ ナ ー ゼ 分 子 の 機 能 効 率 を 低 下 さ せ る も の で あ る と 考 え ら れ て い る [ 6 2 ] 。
高 イ ン ス リ ン 血 性 低 血 糖 症 [ 編 集 ] 一 部 の 変 異 は イ ン ス リ ン の 分 泌 を 増 加 さ せ る こ と が 判 明 し て い る 。 こ う し た 機 能 獲 得 型 変 異 の ヘ テ ロ 接 合 型 で は 、 イ ン ス リ ン 放 出 の 閾 値 が 低 下 す る 。 そ の 結 果 、 一 過 性 ま た は 持 続 性 の 先 天 性 高 イ ン ス リ ン 血 症 や 、 高 齢 で 出 現 す る 反 応 性 低 血 糖 症 な ど 、 さ ま ざ ま な パ タ ー ン の 低 血 糖 症 が 引 き 起 こ さ れ る 。 17 種 類 の G C K の 変 異 が 高 イ ン ス リ ン 血 性 の 低 血 糖 症 を 引 き こ す と さ れ て い る [ 6 2 ] 。
い く つ か の 製 薬 会 社 が グ ル コ キ ナ ー ゼ を 活 性 化 す る 分 子 の 研 究 を 行 っ て い る 。 こ う し た 分 子 は 2 型 糖 尿 病 の 治 療 に 有 用 で あ る 可 能 性 が あ る [ 6 3 ] [ 6 4 ] [ 6 5 ] 。
^ a b c GRCm38: Ensembl release 89: ENSMUSG00000041798 - Ensembl , May 2017
^ Human PubMed Reference: ^ Mouse PubMed Reference: ^ “Hypothesis: structures, evolution, and ancestor of glucose kinases in the hexokinase family”. Journal of Bioscience and Bioengineering 99 (4): 320–30. (April 2005). doi :10.1263/jbb.99.320 . PMID 16233797 . ^ a b c d e “Molecular physiology of mammalian glucokinase” . Cellular and Molecular Life Sciences 66 (1): 27–42. (January 2009). doi :10.1007/s00018-008-8322-9 . PMC 2780631 . PMID 18726182 . https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2780631/ .
^ a b “Comparative biochemistry of glucokinase”. Glucokinase And Glycemic Disease: From Basics to Novel Therapeutics (Frontiers in Diabetes) . Basel: S. Karger AG (Switzerland). (2004). pp. 31–41. ISBN 3-8055-7744-3
^ “Cloning and biochemical characterization of a novel mouse ADP-dependent glucokinase”. Biochemical and Biophysical Research Communications 315 (3): 652–8. (March 2004). doi :10.1016/j.bbrc.2004.01.103 . PMID 14975750 . ^ “Glucokinase as a glucose sensor: past, present, and future”. Glucokinase And Glycemic Disease: From Basics to Novel Therapeutics (Frontiers in Diabetes) . Basel: S. Karger AG (Switzerland). (2004). pp. 18–30. ISBN 3-8055-7744-3 ^ a b c d “Glucokinase”. Encyclopedia of Molecular Medicine . Hoboken: John Wiley & Sons. (2002). ISBN 978-0-471-37494-7
^ a b c “Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm”. Diabetes 45 (2): 223–41. (February 1996). doi :10.2337/diabetes.45.2.223 . PMID 8549869 .
^ “Glucose-induced conformational changes in glucokinase mediate allosteric regulation: transient kinetic analysis”. Biochemistry 45 (24): 7553–62. (June 2006). doi :10.1021/bi060253q . PMID 16768451 . ^ “Pancreatic beta-cell glucokinase: closing the gap between theoretical concepts and experimental realities”. Diabetes 47 (3): 307–15. (March 1998). doi :10.2337/diabetes.47.3.307 . PMID 9519733 . ^ “Identification of alkaline pH optimum of human glucokinase because of ATP-mediated bias correction in outcomes of enzyme assays” . Scientific Reports 9 (1): 11422. (August 2019). doi :10.1038/s41598-019-47883-1 . PMC 6684659 . PMID 31388064 . https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6684659/ . ^ Tiedge, M.; Richter, T.; Lenzen, S. (2000-03-15). “Importance of cysteine residues for the stability and catalytic activity of human pancreatic beta cell glucokinase” . Archives of Biochemistry and Biophysics 375 (2): 251–260. doi :10.1006/abbi.1999.1666 . ISSN 0003-9861 . PMID 10700381 . https://www.ncbi.nlm.nih.gov/pubmed/10700381 . ^ “Crystal structures of Escherichia coli ATP-dependent glucokinase and its complex with glucose” . Journal of Bacteriology 186 (20): 6915–27. (October 2004). doi :10.1128/JB.186.20.6915-6927.2004 . PMC 522197 . PMID 15466045 . https://www.ncbi.nlm.nih.gov/pmc/articles/PMC522197/ . ^ “Structural model of human glucokinase in complex with glucose and ATP: implications for the mutants that cause hypo- and hyperglycemia”. Diabetes 48 (9): 1698–705. (September 1999). doi :10.2337/diabetes.48.9.1698 . PMID 10480597 . ^ “Structural basis for allosteric regulation of the monomeric allosteric enzyme human glucokinase”. Structure 12 (3): 429–38. (March 2004). doi :10.1016/j.str.2004.02.005 . PMID 15016359 . "Beautiful structural pictures illustrating the conformational changes and potential regulatory mechanisms" ^ Kabsch, W.; Holmes, K. C. (1995-02). “The actin fold” . FASEB journal: official publication of the Federation of American Societies for Experimental Biology 9 (2): 167–174. doi :10.1096/fasebj.9.2.7781919 . ISSN 0892-6638 . PMID 7781919 . https://www.ncbi.nlm.nih.gov/pubmed/7781919 . ^ “A polymorphic (CA)n repeat element maps the human glucokinase gene (GCK) to chromosome 7p”. Genomics 12 (2): 319–25. (February 1992). doi :10.1016/0888-7543(92)90380-B . PMID 1740341 . ^ “Human glucokinase gene: isolation, characterization, and identification of two missense mutations linked to early-onset non-insulin-dependent (type 2) diabetes mellitus” . Proceedings of the National Academy of Sciences of the United States of America 89 (16): 7698–702. (August 1992). doi :10.1073/pnas.89.16.7698 . PMC 49778 . PMID 1502186 . https://www.ncbi.nlm.nih.gov/pmc/articles/PMC49778/ . ^ “The hexokinase gene family”. Glucokinase And Glycemic Disease: From Basics to Novel Therapeutics (Frontiers in Diabetes) . Basel: S. Karger AG (Switzerland). (2004). pp. 18–30. ISBN 3-8055-7744-3 ^ a b “Differential expression and regulation of the glucokinase gene in liver and islets of Langerhans” . Proceedings of the National Academy of Sciences of the United States of America 86 (20): 7838–42. (October 1989). doi :10.1073/pnas.86.20.7838 . PMC 298166 . PMID 2682629 . https://www.ncbi.nlm.nih.gov/pmc/articles/PMC298166/ .
^ “Transcriptional induction of glucokinase gene by insulin in cultured liver cells and its repression by the glucagon-cAMP system”. The Journal of Biological Chemistry 264 (36): 21824–9. (December 1989). PMID 2557341 . ^ a b Van Schaftingen, E. (1994-09). “Short-term regulation of glucokinase” . Diabetologia 37 Suppl 2 : S43–47. doi :10.1007/bf00400825 . ISSN 0012-186X . PMID 7821739 . https://www.ncbi.nlm.nih.gov/pubmed/7821739 .
^ a b Kim, So-Youn; Kim, Ha-il; Kim, Tae-Hyun; Im, Seung-Soon; Park, Sang-Kyu; Lee, In-Kyu; Kim, Kyung-Sup; Ahn, Yong-Ho (2004-07-16). “SREBP-1c mediates the insulin-dependent hepatic glucokinase expression” . The Journal of Biological Chemistry 279 (29): 30823–30829. doi :10.1074/jbc.M313223200 . ISSN 0021-9258 . PMID 15123649 . https://www.ncbi.nlm.nih.gov/pubmed/15123649 .
^ a b Foretz, M.; Guichard, C.; Ferré, P.; Foufelle, F. (1999-10-26). “Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes” . Proceedings of the National Academy of Sciences of the United States of America 96 (22): 12737–12742. doi :10.1073/pnas.96.22.12737 . ISSN 0027-8424 . PMC 23076 . PMID 10535992 . https://www.ncbi.nlm.nih.gov/pubmed/10535992 .
^ a b Kim, So-Youn; Kim, Ha-il; Kim, Tae-Hyun; Im, Seung-Soon; Park, Sang-Kyu; Lee, In-Kyu; Kim, Kyung-Sup; Ahn, Yong-Ho (2004-07-16). “SREBP-1c mediates the insulin-dependent hepatic glucokinase expression” . The Journal of Biological Chemistry 279 (29): 30823–30829. doi :10.1074/jbc.M313223200 . ISSN 0021-9258 . PMID 15123649 . https://www.ncbi.nlm.nih.gov/pubmed/15123649 .
^ a b “Analysis of upstream glucokinase promoter activity in transgenic mice and identification of glucokinase in rare neuroendocrine cells in the brain and gut”. The Journal of Biological Chemistry 269 (5): 3641–54. (February 1994). PMID 8106409 .
^ “Glucokinase (GCK) mutations in hyper- and hypoglycemia: maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemia of infancy”. Human Mutation 22 (5): 353–62. (November 2003). doi :10.1002/humu.10277 . PMID 14517946 . ^ De Backer, Ivan; Hussain, Sufyan S.; Bloom, Stephen R.; Gardiner, James V. (07 01, 2016). “Insights into the role of neuronal glucokinase” . American Journal of Physiology. Endocrinology and Metabolism 311 (1): E42–55. doi :10.1152/ajpendo.00034.2016 . ISSN 1522-1555 . PMC 4967152 . PMID 27189932 . https://www.ncbi.nlm.nih.gov/pubmed/27189932 . ^ Sorenson, Robert L.; Stout, Laurence E.; Brelje, T. Clark; Jetton, Thomas L.; Matschinsky, Franz M. (2007-06). “Immunohistochemical evidence for the presence of glucokinase in the gonadotropes and thyrotropes of the anterior pituitary gland of rat and monkey” . The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society 55 (6): 555–566. doi :10.1369/jhc.6A7117.2007 . ISSN 0022-1554 . PMID 17283370 . https://www.ncbi.nlm.nih.gov/pubmed/17283370 . ^ Murphy, R.; Tura, A.; Clark, P. M.; Holst, J. J.; Mari, A.; Hattersley, A. T. (2009-01). “Glucokinase, the pancreatic glucose sensor, is not the gut glucose sensor” . Diabetologia 52 (1): 154–159. doi :10.1007/s00125-008-1183-9 . ISSN 1432-0428 . PMID 18974968 . https://www.ncbi.nlm.nih.gov/pubmed/18974968 . ^ a b c Glucokinase and glycemic disease : from basics to novel therapeutics ISBN 1-4175-6491-1 . OCLC 57298619 . https://www.worldcat.org/oclc/57298619
^ Wang, Zhao Yang; Jin, Ling; Tan, Huanran; Irwin, David M. (2013). “Evolution of hepatic glucose metabolism: liver-specific glucokinase deficiency explained by parallel loss of the gene for glucokinase regulatory protein (GCKR)” . PloS One 8 (4): e60896. doi :10.1371/journal.pone.0060896 . ISSN 1932-6203 . PMC 3613411 . PMID 23573289 . https://www.ncbi.nlm.nih.gov/pubmed/23573289 . ^ Iynedjian, P. B.; Marie, S.; Gjinovci, A.; Genin, B.; Deng, S. P.; Buhler, L.; Morel, P.; Mentha, G. (1995-05). “Glucokinase and cytosolic phosphoenolpyruvate carboxykinase (GTP) in the human liver. Regulation of gene expression in cultured hepatocytes” . The Journal of Clinical Investigation 95 (5): 1966–1973. doi :10.1172/JCI117880 . ISSN 0021-9738 . PMC 295767 . PMID 7738162 . https://www.ncbi.nlm.nih.gov/pubmed/7738162 . ^ Fernández-Novell, J. M.; Bellido, D.; Vilaró, S.; Guinovart, J. J. (1997-01-01). “Glucose induces the translocation of glycogen synthase to the cell cortex in rat hepatocytes” . The Biochemical Journal 321 ( Pt 1) : 227–231. doi :10.1042/bj3210227 . ISSN 0264-6021 . PMC 1218058 . PMID 9003423 . https://www.ncbi.nlm.nih.gov/pubmed/9003423 . ^ Jetton, T. L.; Shiota, M.; Knobel, S. M.; Piston, D. W.; Cherrington, A. D.; Magnuson, M. A. (2001). “Substrate-induced nuclear export and peripheral compartmentalization of hepatic glucokinase correlates with glycogen deposition” . International Journal of Experimental Diabetes Research 2 (3): 173–186. doi :10.1155/edr.2001.173 . ISSN 1560-4284 . PMC 2478546 . PMID 12369705 . https://www.ncbi.nlm.nih.gov/pubmed/12369705 . ^ Aiston, Susan; Andersen, Birgitte; Agius, Loranne (2003-06). “Glucose 6-phosphate regulates hepatic glycogenolysis through inactivation of phosphorylase” . Diabetes 52 (6): 1333–1339. doi :10.2337/diabetes.52.6.1333 . ISSN 0012-1797 . PMID 12765941 . https://www.ncbi.nlm.nih.gov/pubmed/12765941 . ^ Liang, Guosheng; Yang, Jian; Horton, Jay D.; Hammer, Robert E.; Goldstein, Joseph L.; Brown, Michael S. (2002-03-15). “Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c” . The Journal of Biological Chemistry 277 (11): 9520–9528. doi :10.1074/jbc.M111421200 . ISSN 0021-9258 . PMID 11782483 . https://www.ncbi.nlm.nih.gov/pubmed/11782483 . ^ Wu, Chaodong; Okar, David A.; Stoeckman, Angela K.; Peng, Li-Jen; Herrera, Amy H.; Herrera, Julio E.; Towle, Howard C.; Lange, Alex J. (2004-02). “A potential role for fructose-2,6-bisphosphate in the stimulation of hepatic glucokinase gene expression” . Endocrinology 145 (2): 650–658. doi :10.1210/en.2003-1290 . ISSN 0013-7227 . PMID 14617577 . https://www.ncbi.nlm.nih.gov/pubmed/14617577 . ^ Wu, Chaodong; Khan, Salmaan A.; Peng, Li-Jen; Lange, Alex J. (2006). “Roles for fructose-2,6-bisphosphate in the control of fuel metabolism: beyond its allosteric effects on glycolytic and gluconeogenic enzymes” . Advances in Enzyme Regulation 46 : 72–88. doi :10.1016/j.advenzreg.2006.01.010 . ISSN 0065-2571 . PMID 16860376 . https://www.ncbi.nlm.nih.gov/pubmed/16860376 . ^ a b Iynedjian, P. B.; Jotterand, D.; Nouspikel, T.; Asfari, M.; Pilot, P. R. (1989-12-25). “Transcriptional induction of glucokinase gene by insulin in cultured liver cells and its repression by the glucagon-cAMP system” . The Journal of Biological Chemistry 264 (36): 21824–21829. ISSN 0021-9258 . PMID 2557341 . https://www.ncbi.nlm.nih.gov/pubmed/2557341 .
^ Iynedjian, P. B.; Gjinovci, A.; Renold, A. E. (1988-01-15). “Stimulation by insulin of glucokinase gene transcription in liver of diabetic rats” . The Journal of Biological Chemistry 263 (2): 740–744. ISSN 0021-9258 . PMID 3275657 . https://www.ncbi.nlm.nih.gov/pubmed/3275657 . ^ Nelson, Joel D.; LeBoeuf, Renée C.; Bomsztyk, Karol (2011-01). “Direct recruitment of insulin receptor and ERK signaling cascade to insulin-inducible gene loci” . Diabetes 60 (1): 127–137. doi :10.2337/db09-1806 . ISSN 1939-327X . PMC 3012164 . PMID 20929976 . https://www.ncbi.nlm.nih.gov/pubmed/20929976 . ^ a b Langlet, Fanny; Haeusler, Rebecca A.; Lindén, Daniel; Ericson, Elke; Norris, Tyrrell; Johansson, Anders; Cook, Joshua R.; Aizawa, Kumiko et al. (2017-11-02). “Selective Inhibition of FOXO1 Activator/Repressor Balance Modulates Hepatic Glucose Handling” . Cell 171 (4): 824–835.e18. doi :10.1016/j.cell.2017.09.045 . ISSN 1097-4172 . PMC 5687849 . PMID 29056338 . https://www.ncbi.nlm.nih.gov/pubmed/29056338 .
^ Höppner, W.; Seitz, H. J. (1989-12-05). “Effect of thyroid hormones on glucokinase gene transcription in rat liver” . The Journal of Biological Chemistry 264 (34): 20643–20647. ISSN 0021-9258 . PMID 2584235 . https://www.ncbi.nlm.nih.gov/pubmed/2584235 . ^ Chauhan, J.; Dakshinamurti, K. (1991-06-05). “Transcriptional regulation of the glucokinase gene by biotin in starved rats” . The Journal of Biological Chemistry 266 (16): 10035–10038. ISSN 0021-9258 . PMID 2037560 . https://www.ncbi.nlm.nih.gov/pubmed/2037560 . ^ Cabrera-Valladares, G.; German, M. S.; Matschinsky, F. M.; Wang, J.; Fernandez-Mejia, C. (1999-07). “Effect of retinoic acid on glucokinase activity and gene expression and on insulin secretion in primary cultures of pancreatic islets” . Endocrinology 140 (7): 3091–3096. doi :10.1210/endo.140.7.6765 . ISSN 0013-7227 . PMID 10385401 . https://www.ncbi.nlm.nih.gov/pubmed/10385401 . ^ Tippett, P. S.; Neet, K. E. (1982-11-10). “Specific inhibition of glucokinase by long chain acyl coenzymes A below the critical micelle concentration” . The Journal of Biological Chemistry 257 (21): 12839–12845. ISSN 0021-9258 . PMID 7130181 . https://www.ncbi.nlm.nih.gov/pubmed/7130181 . ^ Cárdenas, María Luz (1995). "Glucokinase": Its Regulation and Role in Liver Metabolism (Molecular Biology Intelligence Unit) . R G Landes. ISBN 1-57059-207-1 . "This is the most detailed treatment of liver glucokinase" ^ a b c d Beck, Tobias; Miller, Brian G. (2013-09-10). “Structural basis for regulation of human glucokinase by glucokinase regulatory protein” . Biochemistry 52 (36): 6232–6239. doi :10.1021/bi400838t . ISSN 1520-4995 . PMC 3859847 . PMID 23957911 . https://www.ncbi.nlm.nih.gov/pubmed/23957911 .
^ a b Shiota, C.; Coffey, J.; Grimsby, J.; Grippo, J. F.; Magnuson, M. A. (1999-12-24). “Nuclear import of hepatic glucokinase depends upon glucokinase regulatory protein, whereas export is due to a nuclear export signal sequence in glucokinase” . The Journal of Biological Chemistry 274 (52): 37125–37130. doi :10.1074/jbc.274.52.37125 . ISSN 0021-9258 . PMID 10601273 . https://www.ncbi.nlm.nih.gov/pubmed/10601273 .
^ Farrelly, D.; Brown, K. S.; Tieman, A.; Ren, J.; Lira, S. A.; Hagan, D.; Gregg, R.; Mookhtiar, K. A. et al. (1999-12-07). “Mice mutant for glucokinase regulatory protein exhibit decreased liver glucokinase: a sequestration mechanism in metabolic regulation” . Proceedings of the National Academy of Sciences of the United States of America 96 (25): 14511–14516. doi :10.1073/pnas.96.25.14511 . ISSN 0027-8424 . PMC 24467 . PMID 10588736 . https://www.ncbi.nlm.nih.gov/pubmed/10588736 . ^ Bustamante, Ernesto; Pediaditakis, Peter; He, Lihua; Lemasters, John J. (2005-09-02). “Isolated mouse liver mitochondria are devoid of glucokinase” . Biochemical and Biophysical Research Communications 334 (3): 907–910. doi :10.1016/j.bbrc.2005.06.174 . ISSN 0006-291X . PMID 16036222 . https://www.ncbi.nlm.nih.gov/pubmed/16036222 . ^ Komatsu, Mitsuhisa; Takei, Masahiro; Ishii, Hiroaki; Sato, Yoshihiko (2013-11-27). “Glucose-stimulated insulin secretion: A newer perspective” . Journal of Diabetes Investigation 4 (6): 511–516. doi :10.1111/jdi.12094 . ISSN 2040-1116 . PMC 4020243 . PMID 24843702 . https://www.ncbi.nlm.nih.gov/pubmed/24843702 . ^ Langer, Sara; Kaminski, Martin T.; Lenzen, Sigurd; Baltrusch, Simone (2010-10). “Endogenous activation of glucokinase by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase is glucose dependent” . Molecular Endocrinology (Baltimore, Md.) 24 (10): 1988–1997. doi :10.1210/me.2010-0115 . ISSN 1944-9917 . PMC 5417398 . PMID 20702580 . https://www.ncbi.nlm.nih.gov/pubmed/20702580 . ^ Leibiger, B.; Leibiger, I. B.; Moede, T.; Kemper, S.; Kulkarni, R. N.; Kahn, C. R.; de Vargas, L. M.; Berggren, P. O. (2001-03). “Selective insulin signaling through A and B insulin receptors regulates transcription of insulin and glucokinase genes in pancreatic beta cells” . Molecular Cell 7 (3): 559–570. doi :10.1016/s1097-2765(01)00203-9 . ISSN 1097-2765 . PMID 11463381 . https://www.ncbi.nlm.nih.gov/pubmed/11463381 . ^ Watada, H.; Kajimoto, Y.; Umayahara, Y.; Matsuoka, T.; Kaneto, H.; Fujitani, Y.; Kamada, T.; Kawamori, R. et al. (1996-11). “The human glucokinase gene beta-cell-type promoter: an essential role of insulin promoter factor 1/PDX-1 in its activation in HIT-T15 cells” . Diabetes 45 (11): 1478–1488. doi :10.2337/diab.45.11.1478 . ISSN 0012-1797 . PMID 8866550 . https://www.ncbi.nlm.nih.gov/pubmed/8866550 . ^ Kim, Ha-il; Cha, Ji-Young; Kim, So-Youn; Kim, Jae-woo; Roh, Kyung Jin; Seong, Je-Kyung; Lee, Nam Taek; Choi, Kang-Yell et al. (2002-03). “Peroxisomal proliferator-activated receptor-gamma upregulates glucokinase gene expression in beta-cells” . Diabetes 51 (3): 676–685. doi :10.2337/diabetes.51.3.676 . ISSN 0012-1797 . PMID 11872666 . https://www.ncbi.nlm.nih.gov/pubmed/11872666 . ^ “Glucokinase is an integral component of the insulin granules in glucose-responsive insulin secretory cells and does not translocate during glucose stimulation”. Diabetes 53 (9): 2346–52. (September 2004). doi :10.2337/diabetes.53.9.2346 . PMID 15331544 . ^ Matschinsky, Franz M.; Wilson, David F. (2019). “The Central Role of Glucokinase in Glucose Homeostasis: A Perspective 50 Years After Demonstrating the Presence of the Enzyme in Islets of Langerhans” . Frontiers in Physiology 10 : 148. doi :10.3389/fphys.2019.00148 . ISSN 1664-042X . PMC 6435959 . PMID 30949058 . https://www.ncbi.nlm.nih.gov/pubmed/30949058 . ^ a b “Evidence-based tailoring of bioinformatics approaches to optimize methods that predict the effects of nonsynonymous amino acid substitutions in glucokinase” . Scientific Reports 7 (1): 9499. (August 2017). doi :10.1038/s41598-017-09810-0 . PMC 5573313 . PMID 28842611 . https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5573313/ .
^ “Glucokinase activators in diabetes management”. Expert Opinion on Investigational Drugs 17 (2): 145–67. (February 2008). doi :10.1517/13543784.17.2.145 . PMID 18230050 . ^ “Assessing the potential of glucokinase activators in diabetes therapy”. Nature Reviews. Drug Discovery 8 (5): 399–416. (May 2009). doi :10.1038/nrd2850 . PMID 19373249 . ^ “A patent review of glucokinase activators and disruptors of the glucokinase--glucokinase regulatory protein interaction: 2011-2014”. Expert Opinion on Therapeutic Patents 24 (8): 875–91. (August 2014). doi :10.1517/13543776.2014.918957 . PMID 24821087 .
関連文献 [ 編集 ] 外部リンク [ 編集 ]
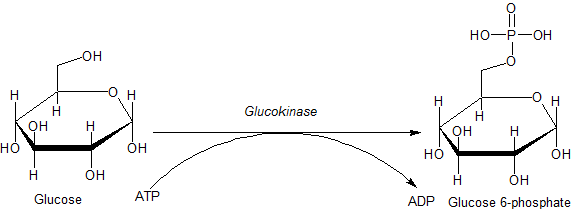 ATPはマグネシウム︵Mg︶を補因子として結合した複合体として反応に参加する。さらに特定の条件下では、グルコキナーゼは他のヘキソキナーゼと同様、他のヘキソースや類似物質のリン酸化を誘導することができる。そのためより正確には、グルコキナーゼが触媒する一般的反応は次のように記述される[6]。
Hexose + MgATP2− → Hexose-PO2−
3 + MgADP− + H+
基質となりうるヘキソースにはマンノース、フルクトース、グルコサミンなどがあるが、これらのヘキソースに対する親和性は低く、十分な活性を示すためには細胞内ではみられないほどの高濃度の基質を必要とする[8]。
ATPはマグネシウム︵Mg︶を補因子として結合した複合体として反応に参加する。さらに特定の条件下では、グルコキナーゼは他のヘキソキナーゼと同様、他のヘキソースや類似物質のリン酸化を誘導することができる。そのためより正確には、グルコキナーゼが触媒する一般的反応は次のように記述される[6]。
Hexose + MgATP2− → Hexose-PO2−
3 + MgADP− + H+
基質となりうるヘキソースにはマンノース、フルクトース、グルコサミンなどがあるが、これらのヘキソースに対する親和性は低く、十分な活性を示すためには細胞内ではみられないほどの高濃度の基質を必要とする[8]。
 ATPはマグネシウム︵Mg︶を補因子として結合した複合体として反応に参加する。さらに特定の条件下では、グルコキナーゼは他のヘキソキナーゼと同様、他のヘキソースや類似物質のリン酸化を誘導することができる。そのためより正確には、グルコキナーゼが触媒する一般的反応は次のように記述される[6]。
Hexose + MgATP2− → Hexose-PO2−
3 + MgADP− + H+
基質となりうるヘキソースにはマンノース、フルクトース、グルコサミンなどがあるが、これらのヘキソースに対する親和性は低く、十分な活性を示すためには細胞内ではみられないほどの高濃度の基質を必要とする[8]。
ATPはマグネシウム︵Mg︶を補因子として結合した複合体として反応に参加する。さらに特定の条件下では、グルコキナーゼは他のヘキソキナーゼと同様、他のヘキソースや類似物質のリン酸化を誘導することができる。そのためより正確には、グルコキナーゼが触媒する一般的反応は次のように記述される[6]。
Hexose + MgATP2− → Hexose-PO2−
3 + MgADP− + H+
基質となりうるヘキソースにはマンノース、フルクトース、グルコサミンなどがあるが、これらのヘキソースに対する親和性は低く、十分な活性を示すためには細胞内ではみられないほどの高濃度の基質を必要とする[8]。





