生化学
表示
(生物化学から転送)
| 生化学 |
|---|
 |
生化学︵せいかがく、英: biochemistry︶または生物化学︵せいぶつかがく、英: biological chemistry︶は、生体内および生物に関連する化学的プロセスを研究する学問である[1]。化学と生物学の下位分野である生化学は、構造生物学、酵素学、代謝学の3つの分野に分けられる。20世紀の最後の数十年間で、生化学はこれらの分野を通じて、生命現象を説明することに成功した。生命科学のほとんどの分野は、生化学的な方法論と研究によって解明され、発展してきた[2]。生化学は、生きた細胞中や細胞間で生体分子に起こる過程を生み出す化学的基盤を理解することに重点を置いており[3]、それにより組織や器官、そして生物の構造と機能をより深く理解するのにつなげている[4]。また生化学は、生物現象の分子機構を研究する分子生物学とも密接に関係する[5]。
生化学は、タンパク質、核酸、炭水化物、脂質などの生体高分子の構造、結合、機能、そして相互作用に大きく関わっている[6]。これらの分子は、細胞の構造を作り、生命機能の多くの役割を担っている[7]。また、細胞の化学的性質は、小分子やイオンの反応にも依存しており、それには、水や金属イオンなどの無機物や、タンパク質合成のためのアミノ酸などの有機物が含まれる[8]。細胞が、化学反応によって環境からエネルギーを取り出す機構は、代謝として知られている。生化学の主な応用分野は、医学、栄養学、そして農業である。医学では生化学者は病気の原因や治療法を[9]、栄養学では健康と幸福を維持する方法や、栄養不足の影響を研究している[10]。農業では土壌や肥料を研究し、作物の栽培、貯蔵、害虫制御の改善を目標としている。生化学は、プリオンなどの複雑な対象を理解する上でも重要である[11]。
歴史[編集]
詳細は「生化学の歴史」を参照

生化学を最も広い意味で捉えると、生物の構成要素や組成、それらがどのように組み立てられて生命が作られているかを研究する学問と見なすことができる。この意味で、生化学の起源は古代ギリシャまでさかのぼることができるが、特定の科学分野としての生化学は、19世紀のいつか、あるいはもう少し前に始まったといえる[12]。生化学の正確な始まりは、焦点を当てる側面によって異なる。18世紀後半にカール・ヴィルヘルム・シェーレが生物から乳酸︵1780年︶[13]やクエン酸︵1784年︶[14]を単離したが、こうした有機化合物は生体からのみ抽出しうるものと考えられていた[15]。1833年にアンセルム・ペイアンが最初の酵素であるジアスターゼ︵現在のアミラーゼ︶を発見したことを主張する人もいれば[16]、1897年にエドゥアルト・ブフナーが無細胞抽出物でアルコール発酵の複雑な生化学過程を最初に証明したことを考える人もいる[17][18][19]。また、ユストゥス・フォン・リービッヒが1842年に発表した﹃Animal chemistry, or, Organic chemistry in its applications to physiology and pathology﹄という、代謝の化学的理論を提示した影響力のある著作や[12]、それ以前の18世紀のアントワーヌ・ラヴォアジエによる発酵と呼吸の研究を挙げる人もいる[20][21]。近代生化学の創始者と呼ばれ、生化学の複雑な層を解明するのに貢献した多くの先駆者には、タンパク質の化学的性質を研究したエミール・フィッシャーや[22]、酵素や生化学の動的性質を研究したフレデリック・ホプキンズが挙げられる[23]。
生化学︵英: biochemistry︶という言葉は、生物学と化学の組み合わせに由来する。1877年、フェリクス・ホッペ=ザイラーが、﹃Zeitschrift für Physiologische Chemie﹄︵現在のBiological Chemistry誌︶の創刊号の序文で、生理化学︵physiological chemistry︶の同義語としてこの言葉︵独: biochemie︶を使用し、この分野に特化した研究機関の設立を提唱した[24][25]。しかし、この言葉は1903年にドイツの化学者カール・ノイベルグが作ったとされることも多く[26][27][28]、またフランツ・ホフマイスターが作ったとする説もある[29]。
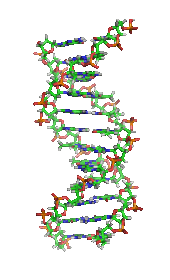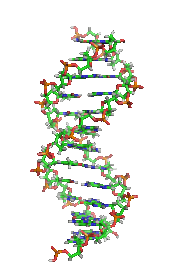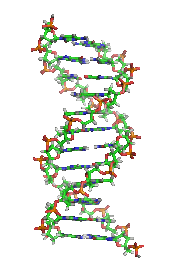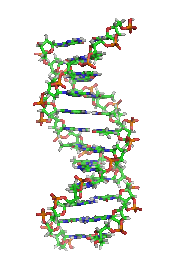
DNAの構造 (1D65)[30]
かつては、生命やその材料には、非生物に見られるものとは異なる本質的な性質や物質があり、生命の分子を作り出せるのは生物だけであると広く信じられていた︵生命原理と呼ばれる︶[31]。1828年、フリードリヒ・ヴェーラーが、シアン酸カリウムと硫酸アンモニウムから尿素を合成した論文は、生命原理を覆し、有機化学を確立したとする見方もある[32][33]。しかし、彼の手によって生気論が死んだとヴェーラー合成を否定する人もいて、論争を巻き起こした[34]。その後、生化学は進歩し、特に20世紀半ば以降、クロマトグラフィー、X線回折、二重偏光干渉法、NMR分光法、放射性同位体標識︵放射性トレーサー︶、電子顕微鏡、分子動力学シミュレーションなどの新しい技術が導入された。これらの技術により、物質を精製したり、解糖やクレブス回路︵クエン酸回路︶のような、多くの細胞内分子や代謝経路の発見と詳細な解析が可能となり、生化学を分子レベルで理解することにつながった。
遺伝子の発見と、細胞内での情報伝達に果たすその役割は、生化学の歴史におけるもうひとつの重要な出来事である。1950年代、ジェームズ・D・ワトソン、フランシス・クリック、ロザリンド・フランクリン、モーリス・ウィルキンスは、DNAの構造を解明し、遺伝情報の伝達との関係を示唆することに貢献した[35]。1958年、ジョージ・ビードルとエドワード・タータムは、菌類において1つの遺伝子が1つの酵素を作り出すことを明らかにし、ノーベル賞を受賞した[36]。1988年には、コリン・ピッチフォークがDNA証拠を使って殺人罪で初めて有罪判決を受け、法医学の発展につながった[37]。最近では、アンドリュー・ファイアーとクレイグ・キャメロン・メローが、遺伝子発現を抑制するRNA干渉︵RNAi︶の役割を発見し、2006年のノーベル賞を共同受賞した[38]。

出発物質:生命の化学的要素[編集]
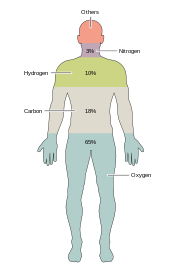
さまざまな種類の生物学的な生命には、約20種類の化学元素が不可欠である。地球上の希少元素の大半︵セレンとヨウ素は除く︶は生命に必要ではなく[39]、アルミニウムやチタンなど豊富に存在する一般的な元素の中には、生命に利用されないものもある。ほとんどの生物は同じような元素を必要とするが、植物と動物には若干の違いがある。たとえば、海洋性藻類は臭素を利用するが、陸上の動物や植物はまったく必要ないようである。また、ナトリウムはすべての動物で必要であるが、植物には必須ではない。逆に、植物にはケイ素とホウ素が必要だが、動物には不要か、あるいは極微量しか必要ない場合がある。
ヒトを含む生体細胞の質量のほぼ99%を、炭素、水素、窒素、酸素、カルシウム、リンのわずか6元素が占めている︵完全な一覧は人体の構成を参照︶。人体の大部分を構成するこれら6種類の主要元素とは別に、ヒトはさらに18種類以上の元素を少量ずつ必要とする[40]。
生体分子[編集]
詳細は「生体分子」を参照
生化学における4種類の主要な分子︵生体分子と呼ばれる︶は、炭水化物、脂質、タンパク質、および核酸である[41]。多くの生体分子はポリマー︵重合体︶である。この文脈ではモノマー︵単量体︶は比較的小さな高分子であり、それらが脱水合成と呼ばれる過程で互いに結合し、生体高分子と呼ばれる大きな高分子を形成している。また、さまざまな高分子が集合して、より大きな複合体を形成することがあり、これは生物学的活性に必要とされることも多い。
炭水化物[編集]
炭水化物は、主にエネルギーの貯蔵と構造の提供という機能を持っている。よく知られている糖類であるグルコースは炭水化物の一つであるが、すべての炭水化物が糖類というわけではない。炭水化物は、地球上に最も多く存在する生体分子であり、エネルギー貯蔵、遺伝情報の保存、細胞間の相互作用やコミュニケーションなど、さまざまな役割を果たしている。
単糖は最も単純な炭水化物で、炭素、水素、酸素を通常は1:2:1の比率で含んでいる︵一般式はCnH2nOn、nは少なくとも3︶。グルコース(C6H12O6)は最も重要な炭水化物であり、その他には甘い果物に含まれるフルクトース(C6H12O6)や、DNAの構成要素であるデオキシリボース(C5H10O4)などがある[42][注釈 1]。単糖には、非環式︵開鎖型︶と環式の状態がある。開鎖型は、一方の端のカルボニル基と他方の端のヒドロキシ基の酸素原子により架橋された炭素原子の環に変化したものである。この環状分子は、直鎖状がアルドースかケトースかによって、ヘミアセタール基かヘミケタール基を持つ[44]。
これらの環状分子は、通常5個または6個の原子を含む環を持ち、それぞれフラノースおよびピラノースと呼ばれる。同様の炭素-酸素環を持つ最も単純な化合物であるフランおよびピラン︵炭素-炭素二重結合を持たない︶に類似していることから、その名が付けられた。たとえば、アルドヘキソースのグルコースは、炭素1の水酸基と炭素4の酸素の間でヘミアセタール結合を形成し、グルコフラノースと呼ばれる5員環の分子を作ることができる。同様の反応は炭素1と炭素5の間でも起こり、グルコピラノースと呼ばれる6員環の分子ができる。7員環のヘプトース (en:英語版) はまれである。
2つの単糖はグリコシド結合またはエステル結合で結合し、脱水反応によって水分子が放出されて二糖になる。二糖のグリコシド結合を切断して2つの単糖に分解する逆の反応を加水分解という。最もよく知られた二糖類はスクロース︵普通の砂糖︶で、グルコース分子とフルクトース分子が結合したものである。もう一つの重要な二糖類は、牛乳に含まれるラクトース︵乳糖︶で、これはグルコース分子とガラクトース分子が結合したものである。乳糖はラクターゼという酵素によって加水分解され、この酵素が欠乏すると乳糖不耐症になる。
単糖が数個︵3-6個程度︶結合したものをオリゴ糖と呼ぶ︵オリゴは﹁少数﹂の意味︶。この分子は、マーカーやシグナルとして使われるなど、さまざまな用途も持っている[45]。単糖が多数結合して多糖を形成する。これらは、1本の長い直鎖で結合することもあれば、分岐した構造になることもある。最も一般的な多糖にはセルロースとグリコーゲンがあり、どちらもグルコースモノマーの繰り返しから構成されている。セルロースは植物の細胞壁の重要な構造成分であり、グリコーゲンは動物のエネルギー源として貯蔵されている。
糖には還元末端または非還元末端がある。炭水化物の還元末端は、開鎖アルデヒド︵アルドース︶またはケト体︵ケトース︶と平衡状態にある炭素原子である。このような炭素原子でモノマーの結合が起こると、ピラノースやフラノース型の遊離ヒドロキシ基が他の糖のOH側鎖と交換され、完全なアセタールが生成される。これにより、アルデヒド型やケト型になることは抑止され、非還元性の修飾残基となる。ラクトースでは、グルコース部分は還元末端であり、ガラクトース部分はグルコースのC4-OH基と完全なアセタールを形成する。サッカロースでは、グルコースのアルデヒド炭素︵C1︶とフルクトースのケト炭素︵C2︶の間で完全なアセタールが形成されるため、還元末端は存在しない。
脂質[編集]
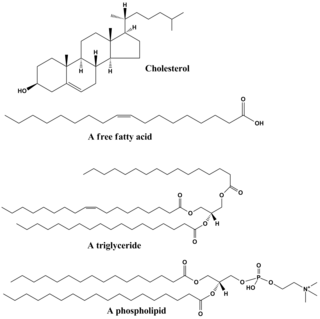
脂質は、生体由来の比較的水に溶けないまたは非極性の化合物グループの総称である。この範ちゅうには、ワックス、脂肪酸、脂肪酸由来のリン脂質、スフィンゴ脂質、糖脂質、およびテルペノイド︵レチノイドやステロイドなど︶などが含まれる。脂質には、直鎖状の脂肪族分子もあれば、環状構造を持つものもある。また、芳香族︵環状と平面状の構造を持つ︶分子もあれば、非芳香族分子もある。脂質には柔軟なものもあれば、硬いものもある。
脂質は通常、グリセロールが他の分子と結合して作られている。バルク脂質の主要なグループであるトリグリセリドは、1分子のグリセロールと3つの脂肪酸が含まれる。ここでいう脂肪酸はモノマーとみなされ、飽和︵炭素鎖に二重結合がない︶または不飽和︵炭素鎖に一つ以上の二重結合がある︶のいずれかになる。
脂質は通常、非極性の部分と極性の部分の両方を持っている。脂質の主な構造は非極性、つまり疎水性︵水をはじく︶であり、水のような極性溶媒とは混ざりにくい。しかし、脂質には極性または親水性︵水になじむ︶の部分もあり、水などの極性溶媒と結合する傾向がある。このため脂質は、疎水性部と親水性部の両方を持つ両親媒性分子となっている。コレステロールを例に取れば、極性基は単なる-OH︵ヒドロキシ基またはアルコール︶である。リン脂質の場合、後述のように、より大きくて極性の強い極性基を持つ。
脂質は、私たちの毎日の食生活を支える重要なものである。バター、チーズ、ギーなど、料理や食事に使う油や乳製品のほとんどは脂肪でできている。植物油には、さまざまな多価不飽和脂肪酸︵PUFA︶が豊富に含まれている。脂質を含む食品は、体内で消化され、最終的な産物である脂肪酸とグリセロールに分解される。脂質、特にリン脂質は、非経口輸液などの共溶解剤として、あるいはリポソームやトランスファソームなどの薬物担体として、さまざまな医薬品にも使用されている。
タンパク質[編集]
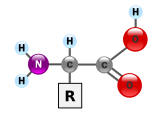
タンパク質は、マクロバイオポリマーとも呼ばれる非常に大きな分子で、アミノ酸というモノマーから構成されている。各アミノ酸は、α炭素原子にアミノ基︵–NH2︶、カルボン酸基︵–COOH、ただし生理学的条件下では–NH3+や–COO−として存在する︶、単一の水素原子、および固有の側鎖︵一般に –R と表記される︶が結合したものである。この側鎖﹁R﹂によって、20種類の標準的なアミノ酸がそれぞれ区別される。この側鎖基﹁R﹂がアミノ酸に異なる性質を与え、タンパク質の全体の立体構造に大きな影響を与える。たとえば神経伝達物質として機能するグルタミン酸のように、単独または修飾された形で機能を持つアミノ酸もある。アミノ酸は、脱水合成という過程でペプチド結合を形成し、互いに結合する。このとき、一方のアミノ酸のアミノ基の窒素と、別のアミノ酸のカルボン酸基の炭素が結びつき、水分子が放出される。こうして作られた分子をジペプチドと呼び、短いアミノ酸の配列︵通常は30個以下︶はペプチドまたはポリペプチド、より長い鎖はタンパク質と呼ばれる。たとえば、血清タンパク質であるアルブミンは、585個のアミノ酸残基から構成されている[48]。

一般的なアミノ酸の構造式を、(1)中性型、(2)生理的に存在する 状態、(3)ジペプチドとして結合した状態で示す。
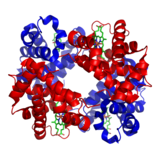
ヘモグロビンの模式図。赤と青のリボンはタンパク質のグロビン、緑の 構造はヘム基を表す。
タンパク質は、構造的な役割と機能的な役割の両方に関与している。たとえば、アクチンとミオシンというタンパク質は、骨格筋の収縮を担っている。多くのタンパク質が持つ特性の1つは、特定の分子または分子群に特異的に結合する能力を持つことである。たとえば、抗体は、特定の1種類の分子に結合するタンパク質である。抗体は、2本の重鎖と2本の軽鎖が、アミノ酸間のジスルフィド結合によって結合して構成されている。抗体は、N末端ドメインの違いにより、標的分子と特異的に結合することができる[49]。
酵素結合免疫吸着法︵ELISA︶は抗体を利用した検査法で、現代医学でがさまざまな生体分子を検出するための最も高感度な方法の一つである。しかし、酵素は最も重要なタンパク質であると考えられている。生細胞内でのほぼすべての反応は、反応の活性化エネルギーを低減させるために酵素が必要である。酵素の分子は、基質と呼ばれる特定の反応分子を識別し、それらの間の反応を触媒することができる[17]。酵素は反応の活性化エネルギーを引き下げることで、その反応速度を1011倍以上に向上させ[17]、通常、自然に起こるのに3,000年以上かかる反応を、1秒以内に起こせる可能性がある[50]。この過程で酵素自体が使い果たされることはなく、新たな一連の基質を用いて同じ反応を触媒し続けることができる。さまざまな修飾剤を用いることで、酵素の活性を調節し、細胞の生化学的な制御を行うことができる[17]。
タンパク質の構造は、慣例で4段階に分類される。一次構造とは、たとえば﹁アラニン-グリシン-トリプトファン-セリン-グルタミン酸-アスパラギン-グリシン-リジン…﹂というように、アミノ酸が一列に並んだ状態のことである。二次構造は、局所的な形態に着目したもので、特定のアミノ酸の組み合わせが、αヘリックスというらせん状に巻きついたり、βシートという板状に折り重なる傾向がある。下の図には、いくつかのαヘリックスをもつヘモグロビンが示されている。三次構造とは、タンパク質の全体的な立体形状を指し、アミノ酸の配列によって決定される。実際、ヘモグロビンのα鎖には146個のアミノ酸残基が含まれ、その6位のグルタミン酸残基がバリン残基に置換された鎌状赤血球症のように、配列の一つの変えると構造全体が変わることがある。四次構造は、4つのサブユニットを持つヘモグロビンのように、複数のペプチドサブユニットを持つタンパク質の構造を扱っている。すべてのタンパク質が複数のサブユニットを持つわけではない[51]。
蛋白質構造データバンクからのタンパク質構造の例。

タンパク質群のメンバーを示す︵イソメラーゼ ドメインのみを示す︶。
摂取されたタンパク質は、通常、小腸で個々のアミノ酸やジペプチドに分解され、体内に吸収される。その後、再び組み合わされて新しいタンパク質が作られる。アミノ酸は、解糖、クエン酸回路、ペントースリン酸経路の中間生成物を使用して作られる。ほとんどの細菌や植物は、20種類すべてのアミノ酸を作るのに必要な酵素を持っている。しかし、ヒトをはじめとする哺乳類は一部の酵素を持たないため、イソロイシン、ロイシン、リシン、メチオニン、フェニルアラニン、トレオニン、トリプトファン、バリンを作ることができない。これらは食餌から摂取しなければならないため必須アミノ酸と呼ばれる。哺乳類は、アラニン、アスパラギン、アスパラギン酸、システイン、グルタミン酸、グルタミン、グリシン、プロリン、セリン、チロシンを合成することができ、これらは非必須アミノ酸と呼ぶ。アルギニンやヒスチジンは作ることができるが、成長期の動物には十分な量を産生できないので、必須アミノ酸とされることがある。
アミノ酸からアミノ基を取り除くと、α-ケト酸という炭素骨格が生成する。トランスアミナーゼ︵アミノ基転移酵素︶と呼ばれる酵素は、あるアミノ酸︵α-ケト酸になる︶から別のα-ケト酸︵アミノ酸になる︶へ、アミノ基を容易に転移させることができる。この過程はタンパク質生合成において重要である。多くの生化学的経路では、他の経路からの中間体がα-ケト酸骨格に変換された後、多くの場合、このアミノ基転移によってアミノ基が付加される。その後、アミノ酸が結合してタンパク質が形成されることもある。
タンパク質が分解される際にも、同様の過程で行われる。最初にタンパク質は加水分解され、個々のアミノ酸になる。血液中にアンモニウムイオン︵NH4+︶として存在する遊離アンモニア︵NH3︶は、生物にとって有毒であるため、生物の必要に応じてさまざまな方法で排泄しなければならない。動物では、その必要性に応じて、さまざまな戦術が進化してきた。単細胞生物はアンモニアを環境中に放出する。同様に、硬骨魚類はアンモニアを水中に放出してすばやく希釈する。一般に、哺乳類は尿素回路によってアンモニアを尿素に変換する。
2つのタンパク質が近縁かどうか、換言すれば相同性があるかどうかを判断するために、科学者は配列アラインメントや構造アラインメントなどの手法を使用する。これらのツールは、関連する分子間の相同性を特定するのに役立ち、タンパク質群の進化パターンを形成する以上の意味を持っている。2つのタンパク質の配列がどの程度似ているかを調べることにより、その構造、さらには機能に関する知識を得ることができる。




核酸[編集]

核酸は、細胞核に多く存在する生体高分子群の総称であり、すべての生きた細胞やウイルスで遺伝情報の源として使用されている[2]。核酸は、ヌクレオチドと呼ばれるモノマーから構成された、複雑で高分子量の生化学高分子である。各ヌクレオチドは、含窒素複素環塩基︵プリンまたはピリミジン︶、ペントース糖、およびリン酸基の3つの成分から構成されている[52]。
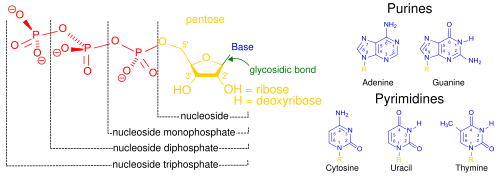
一般的な核酸の構成要素。ヌクレオシド一リン酸、ヌクレオシド二リン 酸、ヌクレオシド三リン酸は、少なくとも一つのリン酸基(赤色)を持つことから、ヌクレオチドと呼ばれる化合物である。(ヌクレオシド(黄色)はリン酸基を持たない)
もっともよく知られている核酸は、デオキシリボ核酸︵DNA︶とリボ核酸︵RNA︶の2種類である。これらの生体高分子では、各ヌクレオチドのリン酸基と糖が結合して骨格を形成し、窒素塩基の配列が遺伝情報の保存を担っている。一般的な窒素塩基は、アデニン、シトシン、グアニン、チミン、ウラシルの5種類である。核酸の鎖に含まれる核酸塩基は、水素結合によって互いに結合し、ジッパーのように相補的な窒素塩基の対を作る。アデニンはチミンまたはウラシルと結合し、チミンはアデニンとのみ、シトシンとグアニンとのみ結合する。ことができる。アデニンとチミン、アデニンとウラシルはそれぞれ2つの水素結合を形成し、シトシンとグアニンの間は3つの水素結合を形成する。
細胞の遺伝物質としての役割に加え、細胞内のセカンドメッセンジャーとしての役割を担うことも多い。また、すべての生物に存在する主要なエネルギー担体分子であるアデノシン三リン酸︵ATP︶の構成要素でもある。RNAとDNAの窒素塩基は異なり、アデニン、シトシン、グアニンは両方に存在し、チミンはDNAにのみ、ウラシルはRNAにのみ存在する。

代謝[編集]
エネルギー源としての炭水化物[編集]
グルコースはほとんどの生命体のエネルギー源である。たとえば、多糖は酵素によってモノマーに分解される(グリコーゲンホスホリラーゼは、多糖であるグリコーゲンからグルコース残基を切断する)。ラクトース(乳糖)やスクロース(ショ糖)などの二糖類は、2つの単糖に切断される。
解糖(嫌気性)[編集]
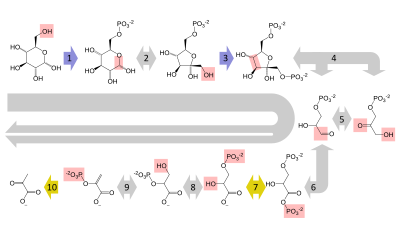
グルコースは主に、解糖という非常に重要な10段階の経路によって代謝され、その結果、1分子のグルコースが2分子のピルビン酸に分解される。また、細胞のエネルギー通貨であるATP︵アデノシン三リン酸︶の正味2分子が生成され、2分子分のNAD+︵酸化型のニコチンアミドアデニンジヌクレオチド︶をNADH︵還元型のニコチンアミドアデニンジヌクレオチド︶に変換する還元当量も生成される。これには酸素を必要としない。酸素がない場合︵あるいは細胞が酸素を使えない場合︶、ピルビン酸を乳酸︵例: ヒト︶またはエタノールと二酸化炭素︵例: 酵母︶に変換することでNADを回復される。ガラクトースやフルクトースなどの他の単糖も、解糖経路の中間体に変換される[53]。
好気性[編集]
ヒトのほとんどの細胞のように、十分な酸素が存在する好気性細胞では、ピルビン酸はさらに代謝される。ピルビン酸は不可逆的にアセチルCoAに変換され、1個の炭素原子が老廃物の二酸化炭素として排出され、別の還元当量としてNADHが生成される。次に、2分子のアセチルCoA︵1分子のグルコースから︶がクエン酸回路に入り、2分子のATP、さらに6分子のNADH、2つの還元型︵ユビ︶キノン︵酵素結合補因子としてFADH2を経由︶を生成し、残りの炭素原子を二酸化炭素として放出する。生成したNAD+とキノール分子は、呼吸鎖の酵素複合体に供給され、電子伝達系が電子を最終的に酸素に伝達し、放出されたエネルギーを生体膜︵真核生物ではミトコンドリア内膜︶を介したプロトン濃度勾配の形で保存する。こうして、酸素は水に還元され、元の電子受容体であるNAD+とキノンが再生される。ヒトが酸素を吸い、二酸化炭素を吐き出すのはこのためである。NADHとキノールの高エネルギー状態から電子が移動することで放出されたエネルギーは、最初にプロトン勾配として蓄えられ、ATPシンターゼ︵合成酵素︶によってATPに変換される。これにより、さらに28分子のATPが生成され︵8つのNADHから24、2つのキノールから4つ︶、分解されたグルコース1分子あたり合計32分子のATPが保存される︵解糖から2つ、クエン酸回路から2つ︶[54]。このように、酸素を使ってグルコースを完全に酸化することは、酸素に依存しない代謝機能よりもはるかに多くのエネルギーを生物に与えることは明らかで、これが、地球の大気に大量の酸素が蓄積された後に複雑な生命が出現した理由であると考えられている。糖新生[編集]
詳細は「糖新生」を参照
脊椎動物では、骨格筋が激しく収縮するとき︵例: 重量挙げや全力疾走のとき︶、エネルギー需要に見合うだけの酸素が供給されないため、グルコースを乳酸に変換するために嫌気性代謝に切り替わる。脂肪やタンパク質などの炭水化物以外からのグルコースが組み合わせ。これは、肝臓のグリコーゲンの貯蔵が枯渇したときにのみ起こる。この経路は、ピルビン酸からグルコースへの解糖の根本的な逆転であり、アミノ酸、グリセロール、クレブス回路︵クエン酸回路︶のような多くの供給源を使用することができる。大規模なタンパク質と脂肪の異化は、通常、飢餓やある種の内分泌疾患に伴って起こる[55]。肝臓は、糖新生と呼ばれる過程を通じてグルコースを再生成する。この過程は解糖と全く逆ではなく、実際には解糖の3倍のエネルギーを必要とする︵解糖では2分子のATPが得られるのに対し、6分子のATPが使用される︶。上記の反応と同様に、生成されたグルコースは、エネルギーを必要とする組織で解糖されたり、グリコーゲン︵植物ではデンプン︶として貯蔵されたり、他の単糖に変換されたり、二糖またはオリゴ糖に結合されたりする。運動中の解糖、血流を介した乳酸の肝臓への移動、その後の糖新生、そして血流へのグルコースの放出という経路を組み合わせたものをコリ回路と呼ぶ[56]。

生化学、遺伝学、分子生物学との関係図。
生化学の研究者は、生化学に特有の技術を使用するが、これらを遺伝学、分子生物学、生物物理学の分野で開発された技術や考え方と組み合わせることも多くなっている。これらの分野の間に明確な境界線はない。生化学は分子の生物学的活性に必要な化学を研究し、分子生物学は分子の生物学的活性を研究し、遺伝学はゲノムが担う分子の遺伝現象を研究する学問である。このことは、右上の図に示すように、各分野の関係を表す一つの可能性である。
他の﹁分子スケール﹂生物科学との関係[編集]

●生化学︵英: biochemistry︶は、生体内で起こる化学物質と生命現象を研究する学問である。生化学者は、生体分子の役割、機能、および構造に重点を置いている。生物学的過程の背後にある化学の研究や、生物学的に活性な分子の合成は、生化学の応用である。生化学は、原子および分子のレベルでの生命の研究である。
●遺伝学︵英: genetics︶とは、生物における遺伝的な差異がもたらす影響を研究する学問である。多くの場合は、正常な構成要素︵例: 1つの遺伝子︶の欠如から推測することができる。変異体、いわゆる野生型あるいは正常な表現型と比較して1つか複数の機能的構成要素を欠く生物の研究である。遺伝的相互作用︵エピスタシス︶は、このような﹁ノックアウト﹂研究の単純な解釈をしばしば混乱させる。
●分子生物学︵英: molecular biology︶は、分子の合成、修飾、機構、および相互作用に焦点を当てた、生命現象の分子基盤を研究する学問である。遺伝物質がRNAに転写され、さらにタンパク質に翻訳されるという分子生物学のセントラルドグマは、単純化されすぎてはいるものの、この分野を理解するための良い出発点となる。この概念は、RNAの新たな役割の出現によって見直されている。
●化学生物学︵英: chemical biology︶は、小分子に基づく新しいツールを開発し、生体系への影響を最小限に抑えながら、その機能に関する詳細な情報を提供することを目指している。さらに、化学生物学では、生体分子と合成装置との非天然ハイブリッドを作り出すために生体システムを利用している︵たとえば、遺伝子治療や薬剤分子を送達できる空のウイルスキャプシド︶。
生化学実験[編集]
生化学実験はIn vitro実験とも呼ばれるように生体細胞の細胞器官内で生じる生化学反応を、複雑な代謝経路や調節機構から切り離してまさに試験管のなかで再現することで研究が進展してきた。21世紀に入ると標識化技術や測定技術の進歩で生きている細胞内で生化学反応を間接的に追跡することも可能になってきたが、生体組織から目的の成分を分離精製する実験技術は生化学研究においては重要な研究技術である。
一般に消化酵素やホルモンのように分泌型の生体物質でない限りは、酵素や受容体を含めて目的の生体物質は特定の組織細胞の特定の細胞小器官にのみ発現・存在している。したがって、生化学実験は標的組織を多数採集し、そこから目的の生体物質を分離精製するところから始まる。
DNAのように細胞破砕後に、エタノール沈澱するだけで捕集できるものもあるが多くの場合、細胞破砕後に密度勾配法による遠心分離で目的の細胞内器官を密度により選択し捕集する。溶液には塩化セシウムなどが用いられる。この状態では多くの場合、酵素や受容体は細胞膜に取り込まれていたり、膜の二重層に埋め込まれているので、界面活性剤を使って脂質膜と分離︿可溶化﹀する必要がある。
目的の生体高分子の精製は古くは半透膜による透析が行われたが、20世紀後半からはゲル濾過クロマトグラフィーやアフィニティークロマトグラフィーにより目的物を精製することが可能になった[57][58]。
代謝による生体内物質の移動や変化の追跡にはトレーサー物質が利用される。古くから放射性あるいは非放射性同位体を組み込んだ生体内物質が広く利用された。しかし同位体置換した生体内物質を用意することは困難をともない、放射性トレーサーの場合はラジオアイソトープセンターなど専用実験施設が必要な為、今日では抗体染色やELISA法など同位体を使用しないトレーサーが広く利用されている[59]。また、微量機器分析技術の進展によりMALDI法などの質量分析でクロマトグラフィ・スポット︵ピーク︶から直接、標的物質の同定も可能である[60]。
イオンチャネルの研究においては、生体膜にガラスの毛細管を押し当てることで、管内にイオンチャネルを閉じ籠めて生化学実験を行うパッチクランプの実験技術によって上記のように生体成分を分離せずに実験を行う技法も開発された。
1990年代以降には特定の無機イオンに反応して蛍光を発する標識色素やルシフェラーゼ遺伝子を応用した形質導入によって、細胞外から蛍光顕微鏡で発光現象を追跡することで間接的に生化学反応をトレースすることも可能になってきている。
参考項目[編集]
詳細は「生化学の概要」を参照
一覧[編集]
参照項目[編集]
- 宇宙生物学 - 宇宙における生命の起源、初期進化、分布、未来を研究する学問分野
- Biochemistry - アメリカ化学会が発行する生化学分野の学術誌
- Biological Chemistry - 生物化学に特化した査読制の科学雑誌
- 生物物理学 - 生物現象を理解するために物理学で用いられてきたアプローチや手法を応用した学際的な科学研究の分野
- 化学生態学 - 生物間の相互作用に関わる化学物質について研究する学問
- 計算論的生物モデル - 生体系のコンピューターモデリングを目的とするシステム生物学や数理生物学の課題領域
- バイオプラスチック - 再生可能なバイオマス資源から生産されるプラスチック材料
- EC番号 (酵素番号) - 酵素が触媒する化学反応に基づく、酵素の数値的な分類体系
- 代わりの生化学 (en:英語版) - 科学的な可能性は合意されていても、存在が証明されていない生化学の形態
- 国際生化学・分子生物学連合 - 生化学および分子生物学に関わる国際的な非政府組織
- メタボローム - 生体試料中に含まれる小分子化学物質の完全な集合
- メタボロミクス - 細胞の代謝物プロファイルに対する系統的研究
- 分子生物学 - 生体分子の合成、修飾、機構、相互作用など、細胞活動の分子基盤に対する生物学の一分野
- 分子医学 - 疾患の根本的な分子的・遺伝的エラーを特定し修正する分子的介入を開発する医学の一分野
- 植物化学 - 植物に由来する化学物質である植物化学物質を研究する学問
- タンパク質分解 - タンパク質がより小さなポリペプチドやアミノ酸に分解されること
- 小分子 - 低分子量(900ダルトン未満)の有機化合物
- 構造生物学 - 生物の構造をあらゆるレベルの組織で分析することを扱う学問分野
- クエン酸回路 - 糖、脂質、タンパク質に由来するアセチルCoAを酸化して貯蔵エネルギーを放出する一連の化学反応
- 口腔生化学
- 分子生物学 - 細胞生物学 - 生物工学 - 遺伝学 - 生物物理学 - 生物有機化学
- 日本生化学会 - 生化学若い研究者の会
脚注[編集]
注釈[編集]
出典[編集]
- ^ “Biological/Biochemistry”. acs.org. 2014年2月6日閲覧。
- ^ a b Voet (2005), p. 3.
- ^ Karp (2009), p. 2.
- ^ Miller (2012). p. 62.
- ^ Astbury (1961), p. 1124.
- ^ Srinivasan, Bharath (March 2022). “A guide to enzyme kinetics in early drug discovery”. The FEBS Journal. doi:10.1111/febs.16404. ISSN 1742-464X. PMID 35175693.
- ^ Eldra (2007), p. 45.
- ^ Marks (2012), Chapter 14.
- ^ Finkel (2009), pp. 1–4.
- ^ UNICEF (2010), pp. 61, 75.
- ^ Cobb, N. J.; Surewicz, W. K. (2009). “Prion Diseases and Their Biochemical Mechanisms - Nathan J. Cobb and Witold K. Surewicz”. Biochemistry 48 (12): 2574–2585. doi:10.1021/bi900108v. PMC 2805067. PMID 19239250.
- ^ a b Helvoort (2000), p. 81.
- ^ Scheele, Carl Wilhelm (1780). “Om Mjölk och dess syra [About milk and its acid]” (Swedish). Kongliga Vetenskaps Academiens Nya Handlingar (New Proceedings of the Royal Academy of Science) 1: 116–124.
- ^ Scheele, Carl Wilhelm (1784). “Anmärkning om Citron-Saft, samt sätt att crystallisera den samma [Note on lemon juice, as well as ways to crystallize the same]” (Swedish). Kongliga Vetenskaps Academiens Nya Handlingar (New Proceedings of the Royal Academy of Science) 5: 105–109.
- ^ 生化学辞典第2版、p.713 【生化学】
- ^ Hunter (2000), p. 75.
- ^ a b c d Srinivasan, Bharath (2020-09-27). “Words of advice: teaching enzyme kinetics”. The FEBS Journal 288 (7): 2068–2083. doi:10.1111/febs.15537. ISSN 1742-464X. PMID 32981225.
- ^ Hamblin (2005), p. 26.
- ^ Hunter (2000), pp. 96–98.
- ^ Berg (1980), pp. 1–2.
- ^ Holmes (1987), p. xv.
- ^ Feldman (2001), p. 206.
- ^ Rayner-Canham (2005), p. 136.
- ^ Ziesak (1999), p. 169.
- ^ Kleinkauf (1988), p. 116.
- ^ Ben-Menahem (2009), p. 2982.
- ^ Amsler (1986), p. 55.
- ^ Horton (2013), p. 36.
- ^ Kleinkauf (1988), p. 43.
- ^ Edwards (1992), pp. 1161–1173.
- ^ Fiske (1890), pp. 419–20.
- ^ Wöhler, F. (1828). “Ueber künstliche Bildung des Harnstoffs”. Annalen der Physik und Chemie 88 (2): 253–256. Bibcode: 1828AnP....88..253W. doi:10.1002/andp.18280880206. ISSN 0003-3804.
- ^ Kauffman (2001), pp. 121–133.
- ^ Lipman, Timothy O. (August 1964). “Wohler's preparation of urea and the fate of vitalism”. Journal of Chemical Education 41 (8): 452. Bibcode: 1964JChEd..41..452L. doi:10.1021/ed041p452. ISSN 0021-9584.
- ^ Tropp (2012), pp. 19–20.
- ^ Krebs (2012), p. 32.
- ^ Butler (2009), p. 5.
- ^ Chandan (2007), pp. 193–194.
- ^ Cox, Nelson, Lehninger (2008). Lehninger Principles of Biochemistry. Macmillan
- ^ Nielsen (1999), pp. 283–303.
- ^ Slabaugh (2007), pp. 3–6.
- ^ Whiting (1970), pp. 1–31.
- ^ Whiting, G.C. (1970), p. 5.
- ^ Voet (2005), pp. 358–359.
- ^ Varki (1999), p. 17.
- ^ Stryer (2007), p. 328.
- ^ Voet (2005), Ch. 12 Lipids and Membranes.
- ^ Metzler (2001), p. 58.
- ^ Feige, Matthias J.; Hendershot, Linda M.; Buchner, Johannes (2010). “How antibodies fold”. Trends in Biochemical Sciences 35 (4): 189–198. doi:10.1016/j.tibs.2009.11.005. PMC 4716677. PMID 20022755.
- ^ Srinivasan, Bharath (2021-07-16). “A Guide to the Michaelis‐Menten equation: Steady state and beyond” (英語). The FEBS Journal 289 (20): 6086–6098. doi:10.1111/febs.16124. ISSN 1742-464X. PMID 34270860.
- ^ Fromm and Hargrove (2012), pp. 35–51.
- ^ Saenger (1984), p. 84.
- ^ Fromm and Hargrove (2012), pp. 163–180.
- ^ Voet (2005), Ch. 17 Glycolysis.
- ^ A Dictionary of Biology. Oxford University Press. (17 September 2015). ISBN 9780198714378
- ^ Fromm and Hargrove (2012), pp. 183–194.
- ^ Meir Wilchek, Talia Miron (1999). “Thirty years of affinity chromatography”. Reactive, Functional Polymers 41 (1): 263-268. doi:10.1016/S1381-5148(99)00042-5. ISSN 1381-5148.
- ^ André M. Striegel, Wallace W. Yau, Joseph J. Kirkland, Donald D. Bly (2009). Modern Size-Exclusion Liquid Chromatography: Practice of Gel Permeation and Gel Filtration Chromatography, Second Edition. doi:10.1002/9780470442876. ISBN 9780471201724.
- ^ Voller, A., Bidwell, D. E., & Bartlett, A. (1979). The enzyme linked immunosorbent assay (ELISA). A guide with abstracts of microplate applications. Dynatech Europe, Borough House, Rue du Pre..
- ^ Hillenkamp, Franz; Jaskolla, Thorsten W; Karas, Michael (2014). “The MALDI process and method”. MALDI MS. A Practical Guide to Instrumentation, Methods, and Applications, 2nd Ed.(Ed.: F. Hillenkamp, J. Peter-Katalinic), Wiley Blackwell, Weinheim, Germany (Wiley Online Library). doi:10.1002/9783527335961.
参考文献[編集]
●Amsler, Mark (1986). The Languages of Creativity: Models, Problem-solving, Discourse. University of Delaware Press. ISBN 978-0-87413-280-9
●Astbury, W.T. (1961). “Molecular Biology or Ultrastructural Biology ?”. Nature 190 (4781): 1124. Bibcode: 1961Natur.190.1124A. doi:10.1038/1901124a0. PMID 13684868.
●Ben-Menahem, Ari (2009). Historical Encyclopedia of Natural and Mathematical Sciences. Springer. 2982. Bibcode: 2009henm.book.....B. ISBN 978-3-540-68831-0
●Burton, Feldman (2001). The Nobel Prize: A History of Genius, Controversy, and Prestige. Arcade Publishing. ISBN 978-1-55970-592-9
●Butler, John M. (2009). Fundamentals of Forensic DNA Typing. Academic Press. ISBN 978-0-08-096176-7
●Sen, Chandan K.; Roy, Sashwati (2007). “MiRNA: Licensed to Kill the Messenger”. DNA and Cell Biology 26 (4): 193–194. doi:10.1089/dna.2006.0567. PMID 17465885.
●Clarence, Peter Berg (1980). The University of Iowa and Biochemistry from Their Beginnings. ISBN 978-0-87414-014-9
●Edwards, Karen J.; Brown, David G.; Spink, Neil; Skelly, Jane V.; Neidle, Stephen (1992). “Molecular structure of the B-DNA dodecamer d(CGCAAATTTGCG)2 an examination of propeller twist and minor-groove water structure at 2·2Åresolution”. Journal of Molecular Biology 226 (4): 1161–1173. doi:10.1016/0022-2836(92)91059-x. PMID 1518049.
●Eldra P. Solomon; Linda R. Berg; Diana W. Martin (2007). Biology, 8th Edition, International Student Edition. Thomson Brooks/Cole. ISBN 978-0-495-31714-2. オリジナルの2016-03-04時点におけるアーカイブ。
●Fariselli, P.; Rossi, I.; Capriotti, E.; Casadio, R. (2006). “The WWWH of remote homolog detection: The state of the art”. Briefings in Bioinformatics 8 (2): 78–87. doi:10.1093/bib/bbl032. PMID 17003074.
●Fiske, John (1890). Outlines of Cosmic Philosophy Based on the Doctrines of Evolution, with Criticisms on the Positive Philosophy, Volume 1. Boston and New York: Houghton, Mifflin 2015年2月16日閲覧。
●Finkel, Richard; Cubeddu, Luigi; Clark, Michelle (2009). Lippincott's Illustrated Reviews: Pharmacology (4th ed.). Lippincott Williams & Wilkins. ISBN 978-0-7817-7155-9
●Krebs, Jocelyn E.; Goldstein, Elliott S.; Lewin, Benjamin; Kilpatrick, Stephen T. (2012). Essential Genes. Jones & Bartlett Publishers. ISBN 978-1-4496-1265-8
●Fromm, Herbert J.; Hargrove, Mark (2012). Essentials of Biochemistry. Springer. ISBN 978-3-642-19623-2
●Hamblin, Jacob Darwin (2005). Science in the Early Twentieth Century: An Encyclopedia. ABC-CLIO. ISBN 978-1-85109-665-7
●Helvoort, Ton van (2000). Arne Hessenbruch. ed. Reader's Guide to the History of Science. Fitzroy Dearborn Publishing. ISBN 978-1-884964-29-9
●Holmes, Frederic Lawrence (1987). Lavoisier and the Chemistry of Life: An Exploration of Scientific Creativity. University of Wisconsin Press. ISBN 978-0-299-09984-8
●Horton, Derek, ed (2013). Advances in Carbohydrate Chemistry and Biochemistry, Volume 70. Academic Press. ISBN 978-0-12-408112-3
●Hunter, Graeme K. (2000). Vital Forces: The Discovery of the Molecular Basis of Life. Academic Press. ISBN 978-0-12-361811-5
●Karp, Gerald (2009). Cell and Molecular Biology: Concepts and Experiments. John Wiley & Sons. ISBN 978-0-470-48337-4
●Kauffman, George B.; Chooljian, Steven H. (2001). “Friedrich Wöhler (1800–1882), on the Bicentennial of His Birth”. The Chemical Educator 6 (2): 121–133. doi:10.1007/s00897010444a.
●Kleinkauf, Horst; Döhren, Hans von; Jaenicke Lothar (1988). The Roots of Modern Biochemistry: Fritz Lippmann's Squiggle and its Consequences. Walter de Gruyter & Co. p. 116. ISBN 978-3-11-085245-5
●Knowles, J.R. (1980). “Enzyme-Catalyzed Phosphoryl Transfer Reactions”. Annual Review of Biochemistry 49: 877–919. doi:10.1146/annurev.bi.49.070180.004305. PMID 6250450.
●Metzler, David Everett; Metzler, Carol M. (2001). Biochemistry: The Chemical Reactions of Living Cells. 1. Academic Press. ISBN 978-0-12-492540-3
●Miller G; Spoolman Scott (2012). Environmental Science – Biodiversity Is a Crucial Part of the Earth's Natural Capital. Cengage Learning. ISBN 978-1-133-70787-5 2016年1月4日閲覧。
●Nielsen, Forrest H. (1999). “Ultratrace minerals”. In Maurice E. Shils. Modern Nutrition in Health and Disease. Baltimore: Williams & Wilkins. pp. 283–303. hdl:10113/46493
●Peet, Alisa (2012). Marks, Allan; Lieberman Michael A.. eds. Marks' Basic Medical Biochemistry (Lieberman, Marks's Basic Medical Biochemistry) (4th ed.). ISBN 978-1-60831-572-7
●Rayner-Canham, Marelene F.; Rayner-Canham, Marelene; Rayner-Canham, Geoffrey (2005). Women in Chemistry: Their Changing Roles from Alchemical Times to the Mid-Twentieth Century. Chemical Heritage Foundation. ISBN 978-0-941901-27-7
●Rojas-Ruiz, Fernando A.; Vargas-Méndez, Leonor Y.; Kouznetsov, Vladimir V. (2011). “Challenges and Perspectives of Chemical Biology, a Successful Multidisciplinary Field of Natural Sciences”. Molecules 16 (3): 2672–2687. doi:10.3390/molecules16032672. PMC 6259834. PMID 21441869.
●Saenger, Wolfram (1984). Principles of Nucleic Acid Structure. New York: Springer-Verlag. ISBN 978-0-387-90762-8
●Slabaugh, Michael R.; Seager, Spencer L. (2013). Organic and Biochemistry for Today (6th ed.). Pacific Grove: Brooks Cole. ISBN 978-1-133-60514-0
●Sherwood, Lauralee; Klandorf, Hillar; Yancey, Paul H. (2012). Animal Physiology: From Genes to Organisms. Cengage Learning. ISBN 978-0-8400-6865-1
●Stryer L, Berg JM, Tymoczko JL (2007). Biochemistry (6th ed.). San Francisco: W.H. Freeman. ISBN 978-0-7167-8724-2
●Tropp, Burton E. (2012). Molecular Biology (4th ed.). Jones & Bartlett Learning. ISBN 978-1-4496-0091-4
●UNICEF (2010). Facts for life (4th ed.). New York: United Nations Children's Fund. ISBN 978-92-806-4466-1. オリジナルの2022-10-09時点におけるアーカイブ。
●Ulveling, Damien; Francastel, Claire; Hubé, Florent (2011). “When one is better than two: RNA with dual functions”. Biochimie 93 (4): 633–644. doi:10.1016/j.biochi.2010.11.004. PMID 21111023. オリジナルの2022-10-09時点におけるアーカイブ。.
●Varki A, Cummings R, Esko J, Jessica F, Hart G, Marth J (1999). Essentials of glycobiology. Cold Spring Harbor Laboratory Press. ISBN 978-0-87969-560-6
●Voet, D; Voet, JG (2005). Biochemistry (3rd ed.). Hoboken, NJ: John Wiley & Sons Inc.. ISBN 978-0-471-19350-0. オリジナルのSeptember 11, 2007時点におけるアーカイブ。
●Whiting, G.C (1970). “Sugars”. In A.C. Hulme. The Biochemistry of Fruits and their Products. 1. London & New York: Academic Press. ISBN 978-0-12-361201-4
●Ziesak, Anne-Katrin; Cram Hans-Robert (1999). Walter de Gruyter Publishers, 1749–1999. Walter de Gruyter & Co. ISBN 978-3-11-016741-2
●Ashcroft, Steve. “Professor Sir Philip Randle; Researcher into metabolism: [1st Edition]”. Independent. ProQuest 311080685
推薦文献[編集]
- Fruton, Joseph S. Proteins, Enzymes, Genes: The Interplay of Chemistry and Biology. Yale University Press: New Haven, 1999. ISBN 0-300-07608-8
- Keith Roberts, Martin Raff, Bruce Alberts, Peter Walter, Julian Lewis and Alexander Johnson, Molecular Biology of the Cell
- 4th Edition, Routledge, March, 2002, hardcover, 1616 pp. ISBN 0-8153-3218-1
- 3rd Edition, Garland, 1994, ISBN 0-8153-1620-8
- 2nd Edition, Garland, 1989, ISBN 0-8240-3695-6
- Kohler, Robert. From Medical Chemistry to Biochemistry: The Making of a Biomedical Discipline. Cambridge University Press, 1982.
- Maggio, Lauren A.; Willinsky, John M.; Steinberg, Ryan M.; Mietchen, Daniel; Wass, Joseph L.; Dong, Ting (2017). “Wikipedia as a gateway to biomedical research: The relative distribution and use of citations in the English Wikipedia”. PLOS ONE 12 (12): e0190046. Bibcode: 2017PLoSO..1290046M. doi:10.1371/journal.pone.0190046. PMC 5739466. PMID 29267345.
外部リンク[編集]
| 生化学に関する 図書館収蔵著作物 |