

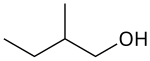
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylbutan-1-ol | |
| Other names
2-Methyl-1-butanol | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.004.809 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H12O | |
| Molar mass | 88.148 g/mol |
| Appearance | colorless liquid |
| Density | 0.8152 g/cm3 |
| Melting point | −117.2 °C (−179.0 °F; 156.0 K) |
| Boiling point | 127.5 °C (261.5 °F; 400.6 K) |
| 31 g/L | |
| Solubility | organic solvents |
| Vapor pressure | 3mm Hg |
| Viscosity | 4.453 mPa·s |
| Thermochemistry | |
Std enthalpy of |
-356.6 kJ·mol−1 (liquid) -301.4 kJ·mol−1 (gas) |
| Hazards | |
| 385 °C (725 °F; 658 K) | |
| Related compounds | |
Related compounds |
Amyl alcohol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
2-Methyl-1-butanol (IUPAC name, also called active amyl alcohol) is an organic compound with the formula CH3CH2CH(CH3)CH2OH. It is one of several isomersofamyl alcohol. This colorless liquid occurs naturally in trace amounts and has attracted some attention as a potential biofuel, exploiting its hydrophobic (gasoline-like) and branched structure. It is chiral.[3]
2-Methyl-1-butanol is a component of many mixtures of commercial amyl alcohols.
2M1B also occurs naturally. For example, fusel alcohols like 2M1B are grain fermentation byproducts, and therefore trace amounts of 2M1B are present in many alcoholic beverages. Also, it is one of the many components of the aroma of various fungi and fruit, e.g., the summer truffle, tomato,[4] and cantaloupe.[5][6]
2-Methyl-1-butanol has been produced from glucose by genetically modified E. coli. 2-Keto-3-methylvalerate, a precursor to threonine, is converted to the target alcohol by the sequential action of 2-keto acid decarboxylase and dehydrogenase.[7] It can be derived from fusel oil (because it occurs naturally in fruits such as grapes[8]) or manufactured by either the oxo process or via the halogenationofpentane.[2]