

| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
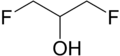
1,3-Difluoropropan-2-ol | |||
| Identifiers | |||
| |||
3D model (JSmol) |
| ||
| ChemSpider |
| ||
| ECHA InfoCard | 100.006.561 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H6F2O | |||
| Molar mass | 96.077 g·mol−1 | ||
| Density | 1.24 g/cm3 (at 25 °C) [1] | ||
| Boiling point | 54 to 55 °C (129 to 131 °F; 327 to 328 K) | ||
| Hazards | |||
| Flash point | 42 °C (108 °F; 315 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
1,3-Difluoro-2-propanol is a metabolic poison which disrupts the citric acid cycle and is used as a rodenticide, similar to sodium fluoroacetate. It is the main ingredient (along with 1-chloro-3-fluoro-2-propanol) in the rodenticide product Gliftor which was widely used in the former USSR[2][3][4] and still approved in China.[5]
|
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anticoagulants / Vitamin K antagonists |
| ||||||||
| Convulsants |
| ||||||||
| Calciferols |
| ||||||||
| Inorganic compounds |
| ||||||||
| Organochlorine |
| ||||||||
| Organophosphorus |
| ||||||||
| Carbamates |
| ||||||||
| Others |
| ||||||||