


| |

| |
| Names | |
|---|---|
| IUPAC name
(2S,3R,4S,5R)-2,3,4,5-Tetrahydroxyhexanedioic acid | |
| Other names
Galactaric acid; Galactosaccharic acid | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.007.641 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H10O8 | |
| Molar mass | 210.138 g·mol−1 |
| Melting point | 230 °C (446 °F; 503 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Mucic acid, C6H10O8 or HOOC-(CHOH)4-COOH (galactaric acidormeso-galactaric acid) is an aldaric acid obtained by nitric acid oxidation of galactose or galactose-containing compounds such as lactose, dulcite, quercite, and most varieties of gum.[1]
Mucic acid forms a crystalline powder, which melts at 210–230 °C.[2] It is insoluble in alcohol, and nearly insoluble in cold water.[1] Due to the symmetry in the molecule, it is optically inactive even though it has chiral carbon atoms (i.e., it is a meso compound).
When heated with pyridine to 140 °C, it is converted into allomucic acid.[1][3] When digested with fuming hydrochloric acid for some time it is converted into αα′ furfural dicarboxylic acid while on heating with barium sulfide it is transformed into α-thiophene carboxylic acid.[1] The ammonium salt yields on dry distillation carbon dioxide, ammonia, pyrrol and other substances.[1] The acid when fused with caustic alkalis yields oxalic acid.[1]
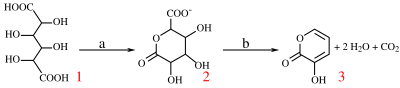
With potassium bisulfate mucic acid forms 3-hydroxy-2-pyrone by dehydration and decarboxylation.
Mucic acid can be used to replace tartaric acidinself-raising flourorfizzies.
It has been used as a precursor of adipic acid in the way to nylon by a rhenium-catalyzed deoxydehydration reaction.[4]
It has been used as a precursor of TaxolinNicolaou Taxol total synthesis (1994).