

| |

| |
| Names | |
|---|---|
| IUPAC name
Bismuth(III) sulfide | |
| Other names
Bismuth sulfide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.014.287 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Bi2S3 | |
| Molar mass | 514.14 g·mol−1 |
| Appearance | brown powder |
| Density | 6.78 g/cm3[1] |
| Melting point | 850 ˚C[1] |
| insoluble | |
| Solubility | soluble in acids |
| -123.0·10−6cm3/mol | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Irritant |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Related compounds | |
Other anions |
Bismuth(III) oxide Bismuth selenide Bismuth telluride |
Other cations |
Arsenic trisulfide Antimony trisulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Bismuth(III) sulfide (Bi2S3) is a chemical compoundofbismuth and sulfur. It occurs in nature as the mineral bismuthinite.
Bismuth(III) sulfide can be prepared by reacting a bismuth(III) salt with hydrogen sulfide:
Bismuth (III) sulfide can also be prepared by the reaction of elemental bismuth and elemental sulfur in an evacuated silica tube at 500 °C for 96 hours.
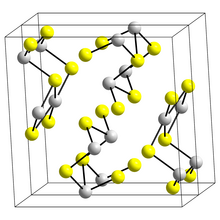
Bismuth(III) sulfide is isostructural with stibnite (stibnite is one of the forms of antimony(III) sulfide). Bismuth atoms are in two different environments, both of which have 7 coordinate Bismuth atoms, 4 in a near planar rectangle and three more distant making an irregular 7-coordination group.[2]
It can react with acids to produce the odoriferous hydrogen sulfide gas.
Bismuth(III) sulfide may be produced in the body by the reaction of the common gastrointestinal drug bismuth subsalicylate with naturally occurring sulfides; this causes temporary black tongue when the sulfides are in the mouth and black feces when the sulfides are in the colon.
It is used as a starting material to produce many other bismuth compounds.[3]
|
| |||
|---|---|---|---|
| Bismuth(III) |
| ||
| Bismuth(V) |
| ||
|
Sulfides (S2−)
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||