

| |
| Names | |
|---|---|
| IUPAC name
Sodium tetrathioantimonate(V) | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.208.207 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Na3SbS4 (anhydrous) Na3SbS4·9H2O (nonahydrate) | |
| Molar mass | 272.13 g·mol−1 (anhydrous) 434.27 g·mol−1 (nonahydrate) |
| Appearance | Yellow crystals |
| Density | 1.806 g/cm3, solid |
| Melting point | 87 °C (189 °F; 360 K) |
| Hazards | |
| GHS labelling: | |
 
| |
| Warning | |
| H302, H332, H411 | |
| P261, P264, P270, P271, P273, P301+P312, P304+P312, P304+P340, P312, P330, P391, P501 | |
| Related compounds | |
Other cations |
Potassium thioantimoniate |
Related compounds |
Antimony(III) sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
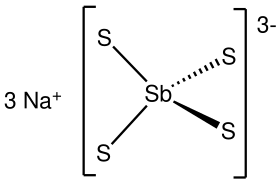
Sodium thioantimoniateorsodium tetrathioantimonate(V) is an inorganic compound with the formula Na3SbS4. The nonahydrate of this chemical, Na3SbS4·9H2O, is known as Schlippe's salt, named after Johann Karl Friedrich von Schlippe (1799–1867). These compounds are examples of sulfosalts. They were once of interest as species generated in qualitative inorganic analysis.

The nonahydrate consists of the tetrahedral tetrathioantimonate(V) anions SbS3−4 and sodium cations Na+, which are hydrated. The Sb-S distance is 2.33 Å.[1][2] Related salts are known for different cations including ammonium and potassium.
The anhydrous salt is a polymer with tetrahedral Na and Sb sites.[3]
Sodium tetrathioantimonate nonahydrate is prepared by the reaction of "antimony trisulfide", elemental sulfur, and aqueous sulfide source.[4]
The Na2S can be generated in situ by the reaction of sodium hydroxide and S (co-generating sodium sulfate):
Charcoal can also be used to reduce the sulfur.
The required antimony trisulfide is prepared by treatment of Sb(III) compounds with sulfide sources:
The hydrate dissolves in water to give the tetrahedral SbS3−4 ion. The salt gives antimony pentasulfide upon acidification:
|
| |||
|---|---|---|---|
| Antimonides |
| ||
| Sb(III) |
| ||
| Sb(III,V) |
| ||
| Sb(V) |
| ||
|
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inorganic |
| ||||||||||||||
| Organic |
| ||||||||||||||