

 | |
 | |
| Clinical data | |
|---|---|
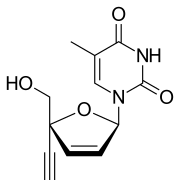
| Other names | 4'-ethynylstavudine, festinavir |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.225.812 |
| Chemical and physical data | |
| Formula | C12H12N2O4 |
| Molar mass | 248.238 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Censavudine (INN;[1] development code BMS-986001) is an investigational new drug being developed by Bristol Myers-Squibb for the treatment of HIV infection.[2][3] It was originally developed at Yale University.[4] It is still in an investigational phase of development as of 2023.[5]
Until 2013, censavudine has been known as festinavir, but the name was changed to avoid confusion with HIV protease inhibitors which all bear class suffix『–navir』(e.g. tipranavir, lopinavir, saquinavir, etc.).[5]
This antiinfective drug article is a stub. You can help Wikipedia by expanding it. |