

 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
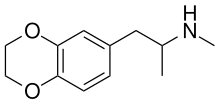
| Formula | C12H17NO2 |
| Molar mass | 207.273 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
3,4-Ethylenedioxy-N-methylamphetamine (EDMA) is an entactogen drug of the methamphetamine class.[1][2] It is an analogueofMDMA where the methylenedioxy ring has been replaced by an ethylenedioxy ring.[1][2] EDMA was first synthesized by Alexander Shulgin.[1] In his book PiHKAL, the dosage is listed as 150–250 mg, and the duration listed as 3–5 hours.[1] According to Shulgin, EDMA produces a bare threshold consisting of paresthesia, nystagmus, and hypnogogic imagery, with few to no other effects.[1] Scientific research has demonstrated that EDMA acts as a non-neurotoxic serotonin releasing agent with moderately diminished potency relative to MDMA, and with negligible effects on dopamine release.[2]
|
| |
|---|---|
| Phenylalkyl- amines (other than cathinones) |
|
| Cyclized phenyl- alkylamines |
|
| Cathinones |
|
| Tryptamines |
|
| Chemical classes |
|
|
| |
|---|---|
| Phenethylamines |
|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines |
|
| Phenylalkylpyrrolidines |
|
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|