

| |
| Names | |
|---|---|
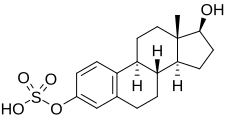
| IUPAC name
17β-Hydroxyestra-1,3,5(10)-trien-3-yl hydrogen sulfate | |
| Systematic IUPAC name
(1S,3aS,3bR,9bS,11aS)-1-Hydroxy-11a-methyl-2,3,3a,3b,4,5,9b,10,11,11a-decahydro-1H-cyclopenta[a]phenanthren-7-yl hydrogen sulfate | |
| Other names
Estra-1,3,5(10)-triene-3,17β-diol 3-sulfate | |
| Identifiers | |
|
| |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H24O5S | |
| Molar mass | 352.445 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Estradiol sulfate (E2S), or 17β-estradiol 3-sulfate,[1] is a natural, endogenous steroid and an estrogen ester.[2] E2S itself is biologically inactive,[3] but it can be converted by steroid sulfatase (also called estrogen sulfatase) into estradiol, which is a potent estrogen.[2][4][5] Simultaneously, estrogen sulfotransferases convert estradiol to E2S, resulting in an equilibrium between the two steroids in various tissues.[2][5] Estrone and E2S are the two immediate metabolic sources of estradiol.[6] E2S can also be metabolized into estrone sulfate (E1S), which in turn can be converted into estrone and estradiol.[7] Circulating concentrations of E2S are much lower than those of E1S.[1] High concentrations of E2S are present in breast tissue, and E2S has been implicated in the biology of breast cancer via serving as an active reservoir of estradiol.[2][4]
As the sodium salt sodium estradiol sulfate, E2S is present as a minor constituent (0.9%) of conjugated equine estrogens (CEEs), or Premarin.[8] It effectively functions as a prodrug to estradiol in this preparation, similarly to E1S. E2S is also formed as a metabolite of estradiol, as well as of estrone and E1S.[9][10] Aside from its presence in CEEs, E2S is not available as a commercial pharmaceutical drug.[11]
E2S shows about 10,000-fold lower potency in activating the estrogen receptors relative to estradiol in vitro.[12] It is 10-fold less potent than estrone sulfate orally in terms of in vivo uterotrophic effect in rats.[13] Estrogen sulfates like estradiol sulfate or estrone sulfate are about twice as potent as the corresponding free estrogens in terms of estrogenic effect when given orally to rodents.[14] This in part led to the introduction of conjugated estrogens (Premarin), which are primarily estrone sulfate, in 1941.[14]
Although inactive at steroid hormone receptors, E2S has been found to act as a potent inhibitorofglutathione S-transferase,[15]anenzyme that contributes to the inactivation of estradiol via conversion of it into an estradiol-glutathione conjugate.[16] As such, E2S can indirectly serve as a positive effector of estrogen signaling.[15]
Estradiol levels are about 1.5- to 4-fold higher than E2S levels in women. This is in contrast to E1S, the levels of which are about 10 to 15 times higher than those of estrone.[17]
E2S at an oral dosage of 5 mg/day in women resulted in inhibition of ovulation in 89% of cycles (47 of 53).[18]
| Estrogen | Other names | RBATooltip Relative binding affinity (%)a | REP (%)b | |||
|---|---|---|---|---|---|---|
| ER | ERα | ERβ | ||||
| Estradiol | E2 | 100 | 100 | 100 | ||
| Estradiol 3-sulfate | E2S; E2-3S | ? | 0.02 | 0.04 | ||
| Estradiol 3-glucuronide | E2-3G | ? | 0.02 | 0.09 | ||
| Estradiol 17β-glucuronide | E2-17G | ? | 0.002 | 0.0002 | ||
| Estradiol benzoate | EB; Estradiol 3-benzoate | 10 | 1.1 | 0.52 | ||
| Estradiol 17β-acetate | E2-17A | 31–45 | 24 | ? | ||
| Estradiol diacetate | EDA; Estradiol 3,17β-diacetate | ? | 0.79 | ? | ||
| Estradiol propionate | EP; Estradiol 17β-propionate | 19–26 | 2.6 | ? | ||
| Estradiol valerate | EV; Estradiol 17β-valerate | 2–11 | 0.04–21 | ? | ||
| Estradiol cypionate | EC; Estradiol 17β-cypionate | ?c | 4.0 | ? | ||
| Estradiol palmitate | Estradiol 17β-palmitate | 0 | ? | ? | ||
| Estradiol stearate | Estradiol 17β-stearate | 0 | ? | ? | ||
| Estrone | E1; 17-Ketoestradiol | 11 | 5.3–38 | 14 | ||
| Estrone sulfate | E1S; Estrone 3-sulfate | 2 | 0.004 | 0.002 | ||
| Estrone glucuronide | E1G; Estrone 3-glucuronide | ? | <0.001 | 0.0006 | ||
| Ethinylestradiol | EE; 17α-Ethynylestradiol | 100 | 17–150 | 129 | ||
| Mestranol | EE 3-methyl ether | 1 | 1.3–8.2 | 0.16 | ||
| Quinestrol | EE 3-cyclopentyl ether | ? | 0.37 | ? | ||
| Footnotes: a = Relative binding affinities (RBAs) were determined via in-vitro displacement of labeled estradiol from estrogen receptors (ERs) generally of rodent uterine cytosol. Estrogen esters are variably hydrolyzed into estrogens in these systems (shorter ester chain length -> greater rate of hydrolysis) and the ER RBAs of the esters decrease strongly when hydrolysis is prevented. b = Relative estrogenic potencies (REPs) were calculated from half-maximal effective concentrations (EC50) that were determined via in-vitro β‐galactosidase (β-gal) and green fluorescent protein (GFP) production assaysinyeast expressing human ERα and human ERβ. Both mammalian cells and yeast have the capacity to hydrolyze estrogen esters. c = The affinities of estradiol cypionate for the ERs are similar to those of estradiol valerate and estradiol benzoate (figure). Sources: See template page. | ||||||
| Estrogen | Structure | Ester(s) | Relative mol. weight |
Relative E2 contentb |
log Pc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Lengtha | ||||||
| Estradiol | – | – | – | – | 1.00 | 1.00 | 4.0 | ||
| Estradiol acetate | C3 | Ethanoic acid | Straight-chain fatty acid | 2 | 1.15 | 0.87 | 4.2 | ||
| Estradiol benzoate | C3 | Benzoic acid | Aromatic fatty acid | – (~4–5) | 1.38 | 0.72 | 4.7 | ||
| Estradiol dipropionate | C3, C17β | Propanoic acid (×2) | Straight-chain fatty acid | 3 (×2) | 1.41 | 0.71 | 4.9 | ||
| Estradiol valerate | C17β | Pentanoic acid | Straight-chain fatty acid | 5 | 1.31 | 0.76 | 5.6–6.3 | ||
| Estradiol benzoate butyrate | C3, C17β | Benzoic acid, butyric acid | Mixed fatty acid | – (~6, 2) | 1.64 | 0.61 | 6.3 | ||
| Estradiol cypionate | C17β | Cyclopentylpropanoic acid | Cyclic fatty acid | – (~6) | 1.46 | 0.69 | 6.9 | ||
| Estradiol enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | 6.7–7.3 | ||
| Estradiol dienanthate | C3, C17β | Heptanoic acid (×2) | Straight-chain fatty acid | 7 (×2) | 1.82 | 0.55 | 8.1–10.4 | ||
| Estradiol undecylate | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.62 | 0.62 | 9.2–9.8 | ||
| Estradiol stearate | C17β | Octadecanoic acid | Straight-chain fatty acid | 18 | 1.98 | 0.51 | 12.2–12.4 | ||
| Estradiol distearate | C3, C17β | Octadecanoic acid (×2) | Straight-chain fatty acid | 18 (×2) | 2.96 | 0.34 | 20.2 | ||
| Estradiol sulfate | C3 | Sulfuric acid | Water-soluble conjugate | – | 1.29 | 0.77 | 0.3–3.8 | ||
| Estradiol glucuronide | C17β | Glucuronic acid | Water-soluble conjugate | – | 1.65 | 0.61 | 2.1–2.7 | ||
| Estramustine phosphated | C3, C17β | Normustine, phosphoric acid | Water-soluble conjugate | – | 1.91 | 0.52 | 2.9–5.0 | ||
| Polyestradiol phosphatee | C3–C17β | Phosphoric acid | Water-soluble conjugate | – | 1.23f | 0.81f | 2.9g | ||
| Footnotes: a = Length of esterincarbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromaticorcyclic fatty acids. b = Relative estradiol content by weight (i.e., relative estrogenic exposure). c = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity/hydrophobicity). Retrieved from PubChem, ChemSpider, and DrugBank. d = Also known as estradiol normustine phosphate. e = Polymerofestradiol phosphate (~13 repeat units). f = Relative molecular weight or estradiol content per repeat unit. g = log P of repeat unit (i.e., estradiol phosphate). Sources: See individual articles. | |||||||||
{{cite book}}: CS1 maint: DOI inactive as of March 2024 (link)
|
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Estrogens |
| ||||||||
| Antiestrogens |
| ||||||||
| |||||||||
|
| |||||||
|---|---|---|---|---|---|---|---|
| ERTooltip Estrogen receptor |
| ||||||
| GPERTooltip G protein-coupled estrogen receptor |
| ||||||
| |||||||