

| |
| Names | |
|---|---|
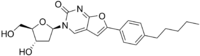
| IUPAC name
3-(2-Deoxy-β-D-erythro-pentofuranosyl)-6-(4-pentylphenyl)furo[2,3-d]pyrimidin-2(3H)-one | |
| Systematic IUPAC name
3-[(2R,4S,5R)-4-Hydroxy-5-(hydroxymethyl)oxolan-2-yl]-6-(4-pentylphenyl)furo[2,3-d]pyrimidin-2(3H)-one | |
| Other names
Cf1743 | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C22H26N2O5 | |
| Molar mass | 398.459 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
FV-100, also known as Cf1743, is an orally available nucleoside analogue drug[1] with antiviral activity.[2] It may be effective against shingles.[3]
It was discovered in 1999 in the laboratories of Prof Chris McGuigan, Welsh School of Pharmacy and Prof. Jan Balzarini, Rega Institute, Leuven, Belgium.[4]
FV-100 was tested against valaciclovir in a phase II trial in patients with herpes zoster. The trial was sponsored by Bristol-Myers Squibb.[5] The drug is currently being developed by ContraVir Pharmaceuticals, Inc., Edison, New Jersey.[6] It has reached Phase III clinical trials.[7]
|
| |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baltimore I |
| ||||||||||||||||||||
| Hepatitis B (VII) |
| ||||||||||||||||||||
| Multiple/general |
| ||||||||||||||||||||
| |||||||||||||||||||||
This antiinfective drug article is a stub. You can help Wikipedia by expanding it. |