

| |
| Names | |
|---|---|
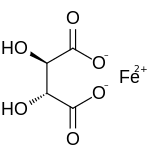
| IUPAC name
(2R,3R)-2,3-dihydroxybutanedioate; iron(2+) | |
| Other names
Iron wine, Ferrous tartrate, Vinum Ferri | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.019.046 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H4FeO6 | |
| Molar mass | 203.92 g/mol |
| Appearance | Reddish powder |
| Pharmacology | |
| B03AA08 (WHO) | |
| |
| Oral | |
| Pharmacokinetics: | |
| yes | |
| Legal status |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ferrous tartrate is a chemical compound and the iron(II) saltoftartaric acid.[1]
Ferrous tartrate has been used as a steel medicine.[2][3] It was generally prescribed during the 19th and early 20th centuries. It is usually prepared by digesting for 30 days, 2 ounces (880 grains) tartarated iron[4] in a pint of sherry.[5] It can be difficult to prepare.[6]
Historically, it was used as a stomachic and tonic, at a dose of 2 tbsp.[5] It was also used to treat anemia, dose 1 to 2 fl. dr.[7]
{{cite book}}: CS1 maint: location missing publisher (link)
|
| |
|---|---|
| Erythropoietins |
|
| Iron supplements |
|
| Vitamin B12 and folic acid supplements |
|
| HIF prolyl-hydroxylase inhibitors |
|
| Other |
|