

| |
| Names | |
|---|---|
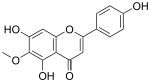
| IUPAC name
4′,5,7-Trihydroxy-6-methoxyflavone | |
| Systematic IUPAC name
5,7-Dihydroxy-2-(4-hydroxyphenyl)-6-methoxy-4H-1-benzopyran-4-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.229.713 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H12O6 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hispidulin is a naturally occurring flavone with potential antiepileptic activity in rats and gerbils.[1][2] It is found in plants including Grindelia argentina, Arrabidaea chica, Saussurea involucrate, Crossostephium chinense, Artemisia, and Salvia.[3]
In traditional and complementary medicine it is claimed to have "antioxidant, antifungal, anti-inflammatory, antimutagenic, and antineoplastic properties".[3]
|
Flavones and their conjugates
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aglycones |
| ||||||||||||
| Glycosides |
| ||||||||||||
| Acetylated |
| ||||||||||||
| Sulfated glycosides | Theograndin I and II | ||||||||||||
| Polymers |
| ||||||||||||
| Drugs |
| ||||||||||||
This article about an aromatic compound is a stub. You can help Wikipedia by expanding it. |