

| |||
| |||
| Names | |||
|---|---|---|---|
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider |
| ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| HI(aq) | |||
| Molar mass | 127.912 g·mol−1 (HI) | ||
| Appearance | colorless liquid | ||
| Odor | acrid | ||
| Density | 1.70 g/mL, azeotrope (57% HI by weight) | ||
| Boiling point | 127 °C (261 °F; 400 K) 1.03 bar, azeotrope | ||
| Aqueous solution | |||
| Acidity (pKa) | −9.3 (HI)[1] | ||
| Hazards | |||
| GHS labelling: | |||

| |||
| Danger | |||
| H314 | |||
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Other anions |
| ||
Related compounds |
Hydrogen iodide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Hydroiodic acid (orhydriodic acid) is a colorless liquid. It is an aqueous solutionofhydrogen iodide with the chemical formula HI(aq). It is a strong acid, in which hydrogen iodide is ionized completely in an aqueous solution. Concentrated aqueous solutions of hydrogen iodide are usually 48% to 57% HI by mass.[2]

Hydroiodic acid reacts with oxygen in air to give iodine:
Like hydrogen halides, hydroiodic acid adds to alkenes to give alkyl iodides. It can also be used as a reducing agent, for example in the reduction of aromatic nitro compoundstoanilines.[3]
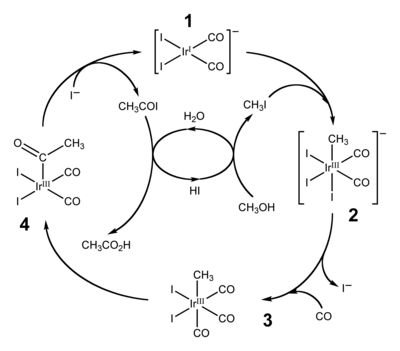
The Cativa process is a major end use of hydroiodic acid, which serves as a co-catalyst for the production of acetic acid by the carbonylationofmethanol.[4][5]
Hydroiodic acid is listed as a U.S. Federal DEA List I Chemical, owing to its use as a reducing agent related to the production of methamphetamine from ephedrineorpseudoephedrine (recovered from nasal decongestant pills).[6]
| Authority control databases: National |
|
|---|