

| |
| Names | |
|---|---|
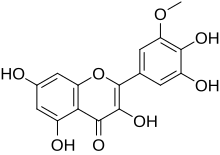
| IUPAC name
3,3′,4′,5,7-Pentahydroxy-5′-methoxyflavone | |
| Systematic IUPAC name
2-(3,4-Dihydroxy-5-methoxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one | |
| Other names
3'-O-Methylmyricetin | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider |
|
| KEGG |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H12O8 | |
| Molar mass | 332.264 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Laricitrin is an O-methylated flavonol, a type of flavonoid. It is found in red grape (absent in white grape)[1] and in Vaccinium uliginosum (bog billberries).[2] It is one of the phenolic compounds present in wine.[3]
Laricitrin is formed from myricetin by the action of the enzyme myricetin O-methyltransferase.[4] It is further methylated by laricitrin 5'-O-methyltransferase into syringetin.