

| |
| Names | |
|---|---|
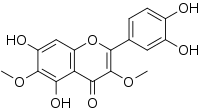
| Preferred IUPAC name
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-3,6-dimethoxy-4H-1-benzopyran-4-one | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C17H14O8 | |
| Molar mass | 346.291 g·mol−1 |
| Density | 1.659 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Axillarin is an O-methylated flavonol. It can be found in Pulicaria crispa, Filifolium sibiricum, Inula britannica,[1] Wyethia bolanderiinBalsamorhiza macrophylla[2] and in Tanacetum vulgare.[3] It can also be synthesized.[4]
Axillarin 7-O-β-D-glucoside can be found in Tagetes mendocina, a plant used in traditional herbal medicine the Andean provinces of Argentina.[5]
{{cite journal}}: CS1 maint: multiple names: authors list (link)
This article about an aromatic compound is a stub. You can help Wikipedia by expanding it. |