

 | |
| Identifiers | |
|---|---|
| |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
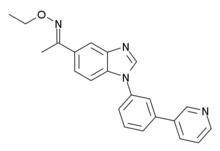
| Formula | C22H20N4O |
| Molar mass | 356.429 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
NS-2710 (LS-193,970) is an anxiolytic drug with a novel chemical structure, developed by the small pharmaceutical company NeuroSearch. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic. NS-2710 is a potent but non-selective partial agonistatGABAA receptors, although with little efficacy at the α1 subtype and more at α2 and α3. It has anxiolytic effects comparable to chlordiazepoxide,[1] and while it is a less potent anticonvulsant than the related drug NS-2664, it has a much longer duration of action, and similarly to other α2/α3-preferring partial agonists produces little sedative effects or physical dependence.[2]
This article about an anxiolytic is a stub. You can help Wikipedia by expanding it. |