

 | |
| Clinical data | |
|---|---|
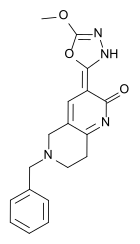
| Other names | 5,6,7,8-tetrahydro-3-(5-methoxy-1,3,4-oxadiazol-2-yl)-6-(phenylmethyl)-1,6-naphthyridin-2(1H)-one |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| Chemical and physical data | |
| Formula | C18H18N4O3 |
| Molar mass | 338.367 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
SX-3228 is a sedative and hypnotic drug used in scientific research. It has similar effects to sedative-hypnotic benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine hypnotic.
SX-3228 is a subtype-selective GABAA positive allosteric modulator acting primarily at the α1 subtype. It thus has similar effects to other α1-selective drugs such as zolpidem and zaleplon in animal studies.[1][2] It only partly substitutes for ethanol, and is a strong sedative-hypnotic with only limited anxiolytic effects which appear only at doses that also produce significant sedation.[3][4]
|
| |
|---|---|
| Alcohols |
|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates |
|
| Flavonoids |
|
| Imidazoles |
|
| Kava constituents |
|
| Monoureides |
|
| Neuroactive steroids |
|
| Nonbenzodiazepines |
|
| Phenols |
|
| Piperidinediones |
|
| Pyrazolopyridines |
|
| Quinazolinones |
|
| Volatiles/gases |
|
| Others/unsorted |
|
See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators | |