J u m p t o c o n t e n t
M a i n m e n u
M a i n m e n u
N a v i g a t i o n
● M a i n p a g e ● C o n t e n t s ● C u r r e n t e v e n t s ● R a n d o m a r t i c l e ● A b o u t W i k i p e d i a ● C o n t a c t u s ● D o n a t e
C o n t r i b u t e
● H e l p ● L e a r n t o e d i t ● C o m m u n i t y p o r t a l ● R e c e n t c h a n g e s ● U p l o a d f i l e
S e a r c h
Search
A p p e a r a n c e
● C r e a t e a c c o u n t ● L o g i n
P e r s o n a l t o o l s
● C r e a t e a c c o u n t ● L o g i n
P a g e s f o r l o g g e d o u t e d i t o r s l e a r n m o r e ● C o n t r i b u t i o n s ● T a l k
( T o p )
1 N a t u r a l o c c u r r e n c e
2 P r o p e r t i e s
3 S y n t h e s i s
4 U s e s
5 H e a l t h a n d s a f e t y
6 S e e a l s o
7 R e f e r e n c e s
T o g g l e t h e t a b l e o f c o n t e n t s
1 - O c t e n - 3 - o l
1 3 l a n g u a g e s
● ا ل ع ر ب ي ة ● ت ۆ ر ک ج ه ● Č e š t i n a ● D e u t s c h ● E s p a ñ o l ● ف ا ر س ی ● F r a n ç a i s ● I t a l i a n o ● M a g y a r ● 日 本 語 ● С р п с к и / s r p s k i ● S r p s k o h r v a t s k i / с р п с к о х р в а т с к и ● 中 文
E d i t l i n k s
● A r t i c l e ● T a l k
E n g l i s h
● R e a d ● E d i t ● V i e w h i s t o r y
T o o l s
T o o l s
A c t i o n s
● R e a d ● E d i t ● V i e w h i s t o r y
G e n e r a l
● W h a t l i n k s h e r e ● R e l a t e d c h a n g e s ● U p l o a d f i l e ● S p e c i a l p a g e s ● P e r m a n e n t l i n k ● P a g e i n f o r m a t i o n ● C i t e t h i s p a g e ● G e t s h o r t e n e d U R L ● D o w n l o a d Q R c o d e ● W i k i d a t a i t e m
P r i n t / e x p o r t
● D o w n l o a d a s P D F ● P r i n t a b l e v e r s i o n
I n o t h e r p r o j e c t s
● W i k i m e d i a C o m m o n s
A p p e a r a n c e
F r o m W i k i p e d i a , t h e f r e e e n c y c l o p e d i a
( R e d i r e c t e d f r o m O c t e n o l )
1-Octen-3-ol
Names
Preferred IUPAC name
Other names
Amyl vinyl carbinol; 1-Vinylhexanol; Matsutake alcohol; Vinyl amyl carbinol; Vinyl hexanol; Matsuica alcohol; Mushroom alcohol; 3-Hydroxy-1-octene; Octenol
Identifiers
CAS Number
3687-48-7 R Y
24587-53-9 S Y
3D model (JSmol )
Interactive image
ChEBI
ChemSpider
ECHA InfoCard 100.020.206
KEGG
PubChem CID
UNII
BYV0MEV7V1 R Y
07D31239FH S Y
CompTox Dashboard (EPA )
InChI=1S/C8H16O/c1-3-5-6-7-8(9 )4-2/h4,8-9H,2-3,5-7H2,1H3 Y
Key: VSMOENVRRABVKN-UHFFFAOYSA-N Y
InChI=1/C8H16O/c1-3-5-6-7-8(9 )4-2/h4,8-9H,2-3,5-7H2,1H3
Key: VSMOENVRRABVKN-UHFFFAOYAB
CCCCCC(C=C)O
OC(C=C)CCCCC
Properties
Chemical formula
C 8 H 16 O
Molar mass
−1
Density
0.837 g/mL
Boiling point
174 ºC at 1 atm
Vapor pressure
0.3 kPa (at 50 °C)
Hazards
GHS labelling
Pictograms
Signal word
Warning
NFPA 704
Flash point
68 ºC
Autoignition
245 ºC
Explosive limits
0.9% (low) to 8% (high)
Lethal dose or concentration (LD, LC):
LD 50 median dose )
340 mg/kg (rat)
Safety data sheet (SDS)
Fisher Scientific
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
Chemical compound
1-Octen-3-ol , octenol for short and also known as mushroom alcohol ,[1] mosquitoes . It is contained in human breath and sweat, and it is believed that insect repellent DEET works by blocking the insects' octenol odorant receptors .[2] [3] [4]
The name “mushroom Alcohol” for 1-octen-3-ol comes from it first isolation by Murahashi in 1936 and 1938 from crushed matsutake mushrooms. [5] [6] [7] antifeedant .[8]
Natural occurrence
[ edit ]
Octenol is produced by several plants and fungi, including edible mushrooms and lemon balm . Octenol is formed during oxidative breakdown of linoleic acid .[9] [10]
It is also a wine fault , defined as a cork taint , occurring in wines made with bunch rot contaminated grape.[11]
Properties
[ edit ]
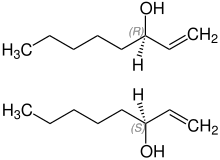
1-octen-3-ol is a secondary alcohol derived from 1-octene . It exists in the form of two enantiomers , (R S
The two enantiomers of 1-octen-3-ol
Synthesis
[ edit ]
Two possible lab syntheses of 1-octen-3-ol are:[12]
Biochemically, 1-octen-3-ol is generated from the peroxidation of linoleic acid , catalyzed by a lipoxygenase , followed by cleavage of the resulting hydroperoxide with the help of a hydroperoxide lyase . This reaction takes place in cheese and is used in biotechnology to produce the (R ) -isomer.[13] [14]
Biosynthesis of (R : 1 2 8 E Z 3 R 4 8 E 5 6
Uses
[ edit ]
Octenol is used, sometimes in combination with carbon dioxide , to attract insects in order to kill them with certain electrical devices.[15]
The name 'mushroom alcohol' is used because octenol is the main flavor component of mushrooms .[16]
Health and safety
[ edit ]
Octenol is approved by the U.S. Food and Drug Administration as a food additive .[17] toxicity with an LD 50 [15]
In an animal study, octenol has been found to disrupt dopamine homeostasis and may be an environmental agent involved in parkinsonism .[18]
See also
[ edit ]
References
[ edit ]
^ Ditzen M, Pellegrino M, Vosshall LB (March 2008). "Insect odorant receptors are molecular targets of the insect repellent DEET". Science . 319 (5871): 1838–42. Bibcode :2008Sci...319.1838D . doi :10.1126/science.1153121 . PMID 18339904 . S2CID 18499590 .
^ Syed Z, Leal WS (September 2008). "Mosquitoes smell and avoid the insect repellent DEET" . Proceedings of the National Academy of Sciences of the United States of America . 105 (36 ): 13598–603. doi :10.1073/pnas.0805312105 PMC 2518096 PMID 18711137 .
^ Murahashi S. "Sci. Pap. Inst. Phys. Chem. Res. (Jpn.) 34, 155". Chemical Absracts . 31
^ Murahashi S. "Sci. Pap. Inst. Phys. Chem. Res. (Jpn.) 30, 263". Chemical Absracts . 32
^ Wood W. F., Lefevre C. K. (2007). "Changing volatile compounds from mycelium and sporocarp of American matsutake mushroom, Tricholoma magnivelare ". Biochemical Systematics and Ecology . 35 9 ): 634–636. Bibcode :2007BioSE..35..634W . doi :10.1016/j.bse.2007.03.001 .
^ Wood WF, Archer CL, Largent DL (2001). "1-Octen-3-ol, a banana slug antifeedant from mushrooms". Biochemical Systematics and Ecology . 29 5 ): 531–533. Bibcode :2001BioSE..29..531W . doi :10.1016/s0305-1978(00 )00076-4 . PMID 11274773 .
^ "Chemical properties of attractants" . Archived from the original on 2009-04-27. Retrieved 2010-06-08 .
^ Bennett JW, Inamdar AA (2015). "Are Some Fungal Volatile Organic Compounds (VOCs) Mycotoxins?" . Toxins . 7 9 ): 3785–3804. doi :10.3390/toxins7093785 PMC 4591661 PMID 26402705 .
^ Steel CC, Blackman JW, Schmidtke LM (June 2013). "Grapevine bunch rots: Impacts on wine composition, quality, and potential procedures for the removal of wine faults". Journal of Agricultural and Food Chemistry . 61 22 ): 5189–206. doi :10.1021/jf400641r . PMID 23675852 .
^ Wnuk S, Kinastowski S, Kamiński E (1983). "Synthesis and analysis of 1-octen-3-ol, the main flavour component of mushrooms" . Die Nahrung . 27 5 ): 479–486. doi :10.1002/food.19830270523 . ISSN 0027-769X . PMID 6684212 .
^ Matsui K, Sasahara S, Akakabe Y, Kajiwara T (2003). "Linoleic acid 10-hydroperoxide as an intermediate during formation of 1-octen-3-ol from linoleic acid in Lentinus decadetes" . Bioscience, Biotechnology, and Biochemistry . 67 10 ): 2280–2282. doi :10.1271/bbb.67.2280 ISSN 0916-8451 . PMID 14586122 . S2CID 46173472 .
^ Min Kuo T, Gardner HW (2002). Lipid biotechnology ISBN 0-585-40371-6 OCLC 48691412 .
^ a b "Biopesticides Fact Sheet for Octenol" (PDF) . EPA fact sheet . 2007-07-05. Retrieved 2022-06-28 .
^ "1-octen-3-ol" . thegoodscentscompany.com . Retrieved 2015-05-31 .
^ US FDAs Center for Food Safety and Applied Nutrition. "US FDA/CFSAN – EAFUS List" . Archived from the original on 2008-02-21. Retrieved 2008-03-16 .
^ Inamdar AA, Hossain MM, Bernstein AI, Miller GW, Richardson JR, Bennett JW (November 2013). "Fungal-derived semiochemical 1-octen-3-ol disrupts dopamine packaging and causes neurodegeneration" . Proceedings of the National Academy of Sciences of the United States of America . 110 (48 ): 19561–6. Bibcode :2013PNAS..11019561I . doi :10.1073/pnas.1318830110 PMC 3845153 PMID 24218591 .
^ Glindemann D, Dietrich A, Staerk HJ, Kuschk P (October 2006). "The two odors of iron when touched or pickled: (skin) carbonyl compounds and organophosphines". Angewandte Chemie . 45 42 ): 7006–9. doi :10.1002/anie.200602100 . PMID 17009284 . S2CID 45055136 .
R e t r i e v e d f r o m " https://en.wikipedia.org/w/index.php?title=1-Octen-3-ol&oldid=1234012613 " C a t e g o r i e s : ● S e c o n d a r y a l c o h o l s ● A l k e n o l s ● V i n y l c o m p o u n d s H i d d e n c a t e g o r i e s : ● C h e m i c a l a r t i c l e s w i t h m u l t i p l e c o m p o u n d I D s ● M u l t i p l e c h e m i c a l s i n a n i n f o b o x t h a t n e e d i n d e x i n g ● C h e m i c a l a r t i c l e s w i t h m u l t i p l e C A S r e g i s t r y n u m b e r s ● E C H A I n f o C a r d I D f r o m W i k i d a t a ● C h e m b o x h a v i n g G H S d a t a ● A r t i c l e s c o n t a i n i n g u n v e r i f i e d c h e m i c a l i n f o b o x e s ● C h e m b o x i m a g e s i z e s e t ● A r t i c l e s w i t h s h o r t d e s c r i p t i o n ● S h o r t d e s c r i p t i o n m a t c h e s W i k i d a t a ● A r t i c l e s w i t h G N D i d e n t i f i e r s
● T h i s p a g e w a s l a s t e d i t e d o n 1 2 J u l y 2 0 2 4 , a t 0 3 : 2 8 ( U T C ) . ● T e x t i s a v a i l a b l e u n d e r t h e C r e a t i v e C o m m o n s A t t r i b u t i o n - S h a r e A l i k e L i c e n s e 4 . 0 ;
a d d i t i o n a l t e r m s m a y a p p l y . B y u s i n g t h i s s i t e , y o u a g r e e t o t h e T e r m s o f U s e a n d P r i v a c y P o l i c y . W i k i p e d i a ® i s a r e g i s t e r e d t r a d e m a r k o f t h e W i k i m e d i a F o u n d a t i o n , I n c . , a n o n - p r o f i t o r g a n i z a t i o n . ● P r i v a c y p o l i c y ● A b o u t W i k i p e d i a ● D i s c l a i m e r s ● C o n t a c t W i k i p e d i a ● C o d e o f C o n d u c t ● D e v e l o p e r s ● S t a t i s t i c s ● C o o k i e s t a t e m e n t ● M o b i l e v i e w