

| |
| Names | |
|---|---|
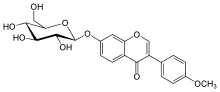
| IUPAC name
7-(β-D-Glucopyranosyloxy)-4′-methoxyisoflavone | |
| Systematic IUPAC name
3-(4-Methoxyphenyl)-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-4H-1-benzopyran-4-one | |
| Other names
Formononetin glucoside | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
| KEGG |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C22H22O9 | |
| Molar mass | 430.409 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Ononin is an isoflavone glycoside, the 7-O-β-D-glucopyranoside of formononetin,[1] which in turn is the 4'-O-methoxy derivative of the parent isoflavone daidzein.
Ononin is a major isoflavone [2] found in a number of plants and herbs like soybean[3] and Glycyrrhiza uralensis.[4]
Intestinal bacterial metabolic pathways may include demethylation and deglycosylation.[5] It follows that formation of formononetin and/or daidzein is possible.
Anin vitro anti-inflammatory effect on lipopolysaccharide (LPS)-induced inflammation has been demonstrated in one study.[6]
{{cite journal}}: CS1 maint: multiple names: authors list (link)
|
Isoflavones and their glycosides
| |
|---|---|
| Isoflavones |
|
| O-methylated isoflavones |
|
| Glycosides |
|
| Prenylated isoflavones |
|
| Pyranoisoflavones |
|
| Derivatives |
|
| Synthetic |
|