J u m p t o c o n t e n t
M a i n m e n u
M a i n m e n u
N a v i g a t i o n
● M a i n p a g e ● C o n t e n t s ● C u r r e n t e v e n t s ● R a n d o m a r t i c l e ● A b o u t W i k i p e d i a ● C o n t a c t u s ● D o n a t e
C o n t r i b u t e
● H e l p ● L e a r n t o e d i t ● C o m m u n i t y p o r t a l ● R e c e n t c h a n g e s ● U p l o a d f i l e
S e a r c h
Search
A p p e a r a n c e
● C r e a t e a c c o u n t ● L o g i n
P e r s o n a l t o o l s
● C r e a t e a c c o u n t ● L o g i n
P a g e s f o r l o g g e d o u t e d i t o r s l e a r n m o r e ● C o n t r i b u t i o n s ● T a l k
( T o p )
1 P r e p a r a t i o n
2 B i o s y n t h e s i s
3 R e a c t i o n s
4 S e e a l s o
5 A d d i t i o n a l r e a d i n g
6 R e f e r e n c e s
T o g g l e t h e t a b l e o f c o n t e n t s
O x a z o l e
2 3 l a n g u a g e s
● ا ل ع ر ب ي ة ● ت ۆ ر ک ج ه ● Č e š t i n a ● D e u t s c h ● E s p a ñ o l ● E s p e r a n t o ● ف ا ر س ی ● F r a n ç a i s ● ह ि न ् द ी ● I t a l i a n o ● L a t v i e š u ● M a g y a r ● N e d e r l a n d s ● 日 本 語 ● P o l s k i ● P o r t u g u ê s ● R o m â n ă ● Р у с с к и й ● С р п с к и / s r p s k i ● S r p s k o h r v a t s k i / с р п с к о х р в а т с к и ● S u o m i ● S v e n s k a ● 中 文
E d i t l i n k s
● A r t i c l e ● T a l k
E n g l i s h
● R e a d ● E d i t ● V i e w h i s t o r y
T o o l s
T o o l s
A c t i o n s
● R e a d ● E d i t ● V i e w h i s t o r y
G e n e r a l
● W h a t l i n k s h e r e ● R e l a t e d c h a n g e s ● U p l o a d f i l e ● S p e c i a l p a g e s ● P e r m a n e n t l i n k ● P a g e i n f o r m a t i o n ● C i t e t h i s p a g e ● G e t s h o r t e n e d U R L ● D o w n l o a d Q R c o d e ● W i k i d a t a i t e m
P r i n t / e x p o r t
● D o w n l o a d a s P D F ● P r i n t a b l e v e r s i o n
I n o t h e r p r o j e c t s
● W i k i m e d i a C o m m o n s
A p p e a r a n c e
F r o m W i k i p e d i a , t h e f r e e e n c y c l o p e d i a
Oxazole
Names
Preferred IUPAC name
Identifiers
CAS Number
3D model (JSmol )
Beilstein Reference
103851
ChEBI
ChEMBL
ChemSpider
ECHA InfoCard 100.005.474
EC Number
Gmelin Reference
485850
MeSH
D010080
PubChem CID
UNII
CompTox Dashboard (EPA )
InChI=1S/C3H3NO/c1-2-5-3-4-1/h1-3H N
Key: ZCQWOFVYLHDMMC-UHFFFAOYSA-N N
InChI=1/C3H3NO/c1-2-5-3-4-1/h1-3H
Key: ZCQWOFVYLHDMMC-UHFFFAOYAD
Properties
Chemical formula
C 3 H 3 N O
Molar mass
69.06 g/mol
Density
1.050 g/cm3
Boiling point
69.5 °C (157.1 °F; 342.6 K )
Acidity (p K a 0.8 (of conjugate acid)[2]
Hazards
GHS labelling[3]
Pictograms
Signal word
Danger
Hazard statements
H225 , H318
Precautionary statements
P210 , P233 , P240 , P241 , P242 , P243 , P264+P265 , P280 , P303+P361+P353 , P305+P354+P338 , P317 , P370+P378 , P403+P235 , P501
Supplementary data page
Oxazole (data page)
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
Chemical compound
Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds . These are azoles with an oxygen and a nitrogen separated by one carbon.[4] aromatic compounds but less so than the thiazoles. Oxazole is a weak base; its conjugate acid has a p K a imidazole .
Preparation [ edit ]
The classic synthetic route the Robinson–Gabriel synthesis by dehydration of 2-acylaminoketones:
The Robinson–Gabriel synthesis
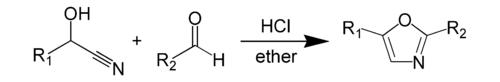
The Fischer oxazole synthesis from cyanohydrins and aldehydes is also widely used:
Fischer Oxazole Synthesis
Other methods are known including the reaction of α-haloketones and formamide and the Van Leusen reaction with aldehydes and TosMIC .
Biosynthesis [ edit ]
In biomolecules , oxazoles result from the cyclization and oxidation of serine or threonine nonribosomal peptides :[5]
Where X = H, CH 3 1 ) Enzymatic cyclization. (2 ) Elimination. (3 ) [O ] = enzymatic oxidation.
Oxazoles are not as abundant in biomolecules as the related thiazoles with oxygen replaced by a sulfur atom.
Reactions [ edit ]
With a pKa pK a Deprotonation of oxazoles occurs at C2. Formylation with dimethylformamide gives 2-formyloxazole. The lithio compound exists in equilibrium with the ring-opened enolate-isonitrile , which can be trapped by silylation .[4]
Electrophilic aromatic substitution takes place at C5, but requiring electron donating groups .
Nucleophilic aromatic substitution takes place with leaving groups at C2.
Diels–Alder reactions involving oxazole (as dienes) and electrophilic alkenes has been well developed as a route to pyridines . In this way, alkoxy-substituted oxazoles serve a precursors to the pyridoxyl system, as found in vitamin B6 . The initial cycloaddition affords a bicyclic intermediate, with an acid-sensitive oxo bridgehead.
Use of an oxazole in the synthesis of a precursor to pyridoxine , which is converted to vitamin B6 .[6]
Cornforth rearrangement of 4-acyloxazoles is a thermal rearrangement reaction with the organic acyl residue and the C5 substituent changing positions.
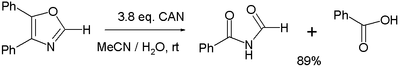
Various oxidation reactions. One study[7] CAN to the corresponding imide and benzoic acid :
In the balanced half-reaction three equivalents of water are consumed for each equivalent of oxazoline, generating 4 protons and 4 electrons (the latter derived from CeIV
See also [ edit ]
Additional reading [ edit ]
Fully Automated Continuous Flow Synthesis of 4,5-Disubstituted Oxazoles Marcus Baumann, Ian R. Baxendale, Steven V. Ley , Christoper D. Smith, and Geoffrey K. Tranmer Org. Lett. ; 2006 ; 8(23 ) pp 5231 - 5234. doi :10.1021/ol061975c
References [ edit ]
^ Zoltewicz, J. A. & Deady, L. W. Quaternization of heteroaromatic compounds. Quantitative aspects. Adv. Heterocycl. Chem. 22, 71-121 (1978).
^ "Oxazole" . pubchem.ncbi.nlm.nih.gov .
^ a b T. L. Gilchrist (1997). Heterocyclic Chemistry (3 ed.). Longman. ISBN 0-582-01421-2
^ Roy, Ranabir Sinha; Gehring, Amy M.; Milne, Jill C.; Belshaw, Peter J.; Walsh, Christopher T.; Roy, Ranabir Sinha; Gehring, Amy M.; Milne, Jill C.; Belshaw, Peter J.; Walsh, Christopher T. (1999). "Thiazole and Oxazole Peptides: Biosynthesis and Molecular Machinery". Natural Product Reports . 16 2 ): 249–263. doi :10.1039/A806930A . PMID 10331285 .
^ Gérard Moine; Hans-Peter Hohmann; Roland Kurth; Joachim Paust; Wolfgang Hähnlein; Horst Pauling; Bernd–Jürgen Weimann; Bruno Kaesler (2011). "Vitamins, 6. B Vitamins". Ullmann's Encyclopedia of Industrial Chemistry doi :10.1002/14356007.o27_o09 . ISBN 978-3-527-30673-2
^ "Ceric Ammonium Nitrate Promoted Oxidation of Oxazoles", David A. Evans , Pavel Nagorny, and Risheng Xu. Org. Lett. 2006 ; 8(24 ) pp 5669 - 5671; (Letter) doi :10.1021/ol0624530
R e t r i e v e d f r o m " https://en.wikipedia.org/w/index.php?title=Oxazole&oldid=1182672747 " C a t e g o r i e s : ● O x a z o l e s ● S i m p l e a r o m a t i c r i n g s H i d d e n c a t e g o r i e s : ● A r t i c l e s w i t h o u t K E G G s o u r c e ● E C H A I n f o C a r d I D f r o m W i k i d a t a ● A r t i c l e s w i t h c h a n g e d I n C h I i d e n t i f i e r ● C h e m b o x h a v i n g G H S d a t a ● C h e m i c a l a r t i c l e s h a v i n g a d a t a p a g e ● A r t i c l e s c o n t a i n i n g u n v e r i f i e d c h e m i c a l i n f o b o x e s ● C h e m b o x i m a g e s i z e s e t ● A r t i c l e s w i t h s h o r t d e s c r i p t i o n ● S h o r t d e s c r i p t i o n m a t c h e s W i k i d a t a
● T h i s p a g e w a s l a s t e d i t e d o n 3 0 O c t o b e r 2 0 2 3 , a t 1 8 : 0 4 ( U T C ) . ● T e x t i s a v a i l a b l e u n d e r t h e C r e a t i v e C o m m o n s A t t r i b u t i o n - S h a r e A l i k e L i c e n s e 4 . 0 ;
a d d i t i o n a l t e r m s m a y a p p l y . B y u s i n g t h i s s i t e , y o u a g r e e t o t h e T e r m s o f U s e a n d P r i v a c y P o l i c y . W i k i p e d i a ® i s a r e g i s t e r e d t r a d e m a r k o f t h e W i k i m e d i a F o u n d a t i o n , I n c . , a n o n - p r o f i t o r g a n i z a t i o n . ● P r i v a c y p o l i c y ● A b o u t W i k i p e d i a ● D i s c l a i m e r s ● C o n t a c t W i k i p e d i a ● C o d e o f C o n d u c t ● D e v e l o p e r s ● S t a t i s t i c s ● C o o k i e s t a t e m e n t ● M o b i l e v i e w