

| |
| Names | |
|---|---|
| Other names
2,3-(S)-hexahydroxydiphenoyl-4,6-(S,S)-gallagyl-D-glucose | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| KEGG | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
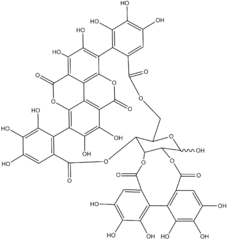
| C48H28O30 | |
| Molar mass | 1084.71 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Punicalagin (Pyuni-cala-jen) is an ellagitannin, a type of phenolic compound. It is found as alpha and beta isomers in pomegranates (Punica granatum), Terminalia catappa, Terminalia myriocarpa,[1] and in Combretum molle, the velvet bushwillow, a plant species found in South Africa.[2] These three genera are all Myrtales and the last two are both Combretaceae.
Punicalagins are water-soluble and hydrolyze into smaller phenolic compounds, such as ellagic acid.
There were no toxic effects in rats on a 6% diet of punicalagins for 37 days.[3] In laboratory research, punicalagins had carbonic anhydrase inhibitor activity.[4]
|
Types of pomegranate ellagitannins
| |
|---|---|
| Aglycones |
|
| Sugars |
|
| Examples |
|