

| |
| Names | |
|---|---|
| IUPAC name
2,2,4,4-tetraoxido-1,5-dioxy-2,3,4-trisulfy-[5]catenate(2−) | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChemSpider | |
| 142337 | |
| KEGG | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
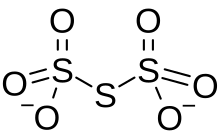
| O6S3−2 | |
| Molar mass | 192.18 g·mol−1 |
| Conjugate acid | Trithionic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Trithionate is an oxyanionofsulfur with the chemical formula S
3O2−
6. It is the conjugate baseoftrithionic acid.[1] Dilute sodium hydroxide hydrolyzes S
4N
4 as follows, yielding sodium thiosulfate and sodium trithionate:
Certain sulfate-reducing bacteria have been known to use the compound in respiration.[2]
This article about chemical compounds is a stub. You can help Wikipedia by expanding it. |