ピルビン酸キナーゼ
ピルビン酸キナーゼ︵ピルビンさんキナーゼ、英: pyruvate kinase︶は、解糖系の最終段階に関与する酵素である。ピルビン酸キナーゼは、ホスホエノールピルビン酸︵PEP︶からアデノシン二リン酸︵ADP︶へのリン酸基の転移を触媒し、1分子のピルビン酸と1分子のATPを生成する[1]。﹁ピルビン酸キナーゼ﹂という名称は、この酵素がピルビン酸のリン酸化を直接触媒しないことが認識される以前に︵一般的なキナーゼとは異なる︶不適切な命名がなされたものであり、この反応は生理的条件下では起こらない[2]。ピルビン酸キナーゼは動物では4種類の組織特異的アイソザイムが存在し、そのそれぞれが多様な組織での代謝要求変動への適応に必要な速度論的特性を持つ。
脊椎動物のアイソザイム
編集
脊椎動物では、ピルビン酸キナーゼはL︵肝臓︶、R︵赤血球︶、M1︵筋肉と脳︶、M2︵初期胎児組織と大部分の成体組織︶の4種類のアイソザイムが発現している。L型とR型のアイソザイムはPKLR遺伝子から発現し、M1、M2型アイソザイムはPKM遺伝子から発現する。L型とR型のアイソザイムには2つの異なるコンフォメーション状態が存在し、一方は基質に対する親和性が高く、もう一方は低い。高い基質親和性で特徴づけられるR状態はピルビン酸キナーゼの活性化型として機能し、PEPとフルクトース-1,6-ビスリン酸︵FBP︶によって安定化されて解糖系経路を促進する。低い基質親和性で特徴づけられるT状態はピルビン酸キナーゼの不活性型として機能し、ATPとアラニンの結合によって安定化されてピルビン酸キナーゼのリン酸化を引き起こし、解糖系を阻害する[3]。M2型アイソザイムは四量体または二量体を形成する。四量体はPEPに対する親和性が高いのに対し、二量体は低い。高活性の四量体型PKM2をリン酸化によって不活性な二量体へ変換することでPKM2の酵素活性は調節される[4]。
PKM遺伝子は12個のエクソンと11個のイントロンから構成される。PKM1とPKM2は選択的スプライシングによる産物であり︵PKM1がエクソン9を含むのに対し、PKM2はエクソン10を含む︶、C末端の56アミノ酸︵378–434番︶のうちの23アミノ酸だけが異なる[5][6]。PKM遺伝子はhnRNPA1やhnRNPA2といったhnRNPによって調節されている[7]。ヒトのPKM2単量体は531アミノ酸からなり、A、B、Cのドメインに分けられる。PKM1とPKM2のアミノ酸配列の差異のため、PKM2はFBPによる調節や二量体・四量体形成による調節が行われるのに対し、PKM1は四量体のみを形成する[8]。
細菌のアイソザイム
編集
大腸菌Escherichia coliなど多くの腸内細菌科の生物にはPykA、PykFの2種類のアイソザイムが存在し、大腸菌では両者の同一性は37%である︵Uniprot: PykA, PykF︶。これらは真核生物のものと同じ反応を触媒し、すなわち解糖系の最終段階でADPとPEPからATPを産生し、この段階は生理的条件下では不可逆的である。PykFはFBPによってアロステリックに調節され、このことは細胞代謝においてPykFが中心的役割を果たしていることを反映している[9]。大腸菌におけるPykFの転写は、グローバルな転写調節因子Cra︵FruR︶によって調節されている[10][11][12]。
反応
編集解糖系
編集
解糖系では、ピルビン酸キナーゼは2段階の反応を触媒する。まず、PEPはADPへリン酸基を転移し、ATPとピルビン酸のエノラートが形成される。続いて、ピルビン酸のエノラートにプロトンが付加され、細胞が必要とする機能的なピルビン酸が形成される[13]。ピルビン酸キナーゼの基質は単純な糖リン酸、そして反応産物はATPであるため、ピルビン酸キナーゼは解糖サイクルの進化の基礎となった酵素である可能性があり、地球上の全ての生命でみられる最も古い酵素の1つである可能性がある。ホスホエノールピルビン酸は非生物的過程によって存在していた可能性があり、また原始的なトリオース解糖系経路において高収率で産生されることが示されている[14]。
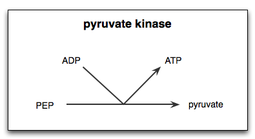
解糖系の最終段階を示した模式図。ピルビン酸キナーゼによってホス ホエノールピルビン酸︵PEP︶からアデノシン二リン酸︵ADP︶へリン酸基が転移され、1分子のピルビン酸と1分子のATPが生成される
酵母細胞では、酵母ピルビン酸キナーゼ︵YPK︶とPEPやアロステリックエフェクターであるFBPとの相互作用は、Mg2+の存在下で強化されることが示されている。そのため、Mg2+はピルビン酸キナーゼによるPEPからピルビン酸への触媒の重要な補因子であると結論付けられる。さらに、Mn2+はYPKに対して同様かつより強い効果を持つことが示されている。ピルビン酸キナーゼの金属結合部位への金属イオンの結合は、この反応を加速させる[15]。
ピルビン酸キナーゼによって触媒される反応は、解糖系の最終段階である。この反応はこの経路の3つの律速段階のうちの1つである。律速段階はある経路の中でより遅く、そして調節を受ける段階であり、そのためこの段階によってその経路全体の速度が決定される。解糖系の律速段階はATPの加水分解またはADPのリン酸化のいずれかと共役しており、そのためこの経路はエネルギー的に有利かつ細胞内で本質的に不可逆なものとなっている。ピルビン酸は他の代謝経路の重要な中間体となるビルディングブロックでもあるため、この最終段階は高度な調節を受け、かつ不可逆なものとなっている[16]。産生されたピルビン酸は、好気条件下でさらなるATP産生のためにTCA回路に入るか、または嫌気条件下で乳酸またはエタノールへ変換される。

糖新生
編集
ピルビン酸キナーゼは糖新生の調節酵素としても機能する。糖新生は、肝臓などでピルビン酸やその他の基質からグルコースを生成する生化学経路である。糖新生は、グルコースの直接的な貯蔵が尽きた際に、非炭水化物を利用してグルコースを脳や赤血球へ供給する[16]。絶食時にはピルビン酸キナーゼは阻害され、ホスホエノールピルビン酸からピルビン酸への変換が防がれる[16]。そしてその代わりに、ホスホエノールピルビン酸は糖新生反応カスケードによってグルコースへと変換される。糖新生は解糖系と類似した酵素が利用されるものの、解糖系の逆反応であるわけではなく、解糖系の不可逆段階を回避する経路である。さらに、細胞内で糖新生と解糖系は細胞シグナル伝達によって相反する調節を受けるため、いかなる時にも両者が同時に行われることはない[16]。糖新生経路が完了すると、産生されたグルコースは肝臓から排出され、重要な組織にエネルギーを供給する。
調節
編集
解糖系は、ヘキソキナーゼによるグルコースのリン酸化、ホスホフルクトキナーゼによるフルクトース-6-リン酸のリン酸化、ピルビン酸キナーゼによるPEPからADPへのリン酸基の転移の3つの触媒段階で高度な調節を受ける。通常の条件下では、これら3つの反応は全て大きな負の自由エネルギーを有する不可逆的反応であり、この経路の調節を担う[16]。ピルビン酸キナーゼの活性は、アロステリックエフェクター、共有結合修飾、ホルモンによって最も広範な調節を受けている。ピルビン酸キナーゼの最も重要な調節因子はフルクトース-1,6-ビスリン酸︵FBP︶であり、この酵素のアロステリックエフェクターとして作用する。
アロステリックエフェクター
編集
アロステリック調節は、エフェクター分子がタンパク質の活性部位以外の場所に結合することで、コンフォメーションや活性の変化を引き起こす調節機構である。ピルビン酸キナーゼはFBPによってアロステリックに活性化され、ATPとアラニンによってアロステリックに不活性化されることが知られている[17]。ピルビン酸キナーゼの四量体化はFBPとセリンによって促進され、四量体の解離はL-システインによって促進される[18][19][20]。
フルクトース-1,6-ビスリン酸
編集
FBPは解糖系経路に由来する、最も重要な調節因子である。FBPはフルクトース-6-リン酸のリン酸化によって産生される、解糖系の中間体である。FBPはピルビン酸キナーゼのドメインCに位置するアロステリック結合部位に結合して酵素のコンフォメーションを変化させ、ピルビン酸キナーゼ活性の活性化を引き起こす[21]。FBPは解糖系経路の中間体であるため、FBPの濃度が高くなるほどピルビン酸キナーゼ活性のアロステリック活性化が大きくなる、フィードフォワード刺激が行われる。ピルビン酸キナーゼはFBPの効果に対する感受性が最も高い。そのため、残りの調節機構は二次的機構である[9][22]。
共有結合修飾
編集
共有結合修飾酵素は酵素のリン酸化、脱リン酸化、アセチル化、スクシニル化、酸化の間接的な調節因子として機能し、酵素活性の活性化や阻害をもたらす[23]。肝臓では、グルカゴンとアドレナリンはプロテインキナーゼA︵PKA︶を活性化し、PKAはピルビン酸キナーゼをリン酸化して不活性化する。対照的に、血糖値の上昇に応答して分泌されるインスリンはプロテインホスファターゼ1︵PP1︶を活性化し、ピルビン酸キナーゼの脱リン酸化による活性化を引き起こす。同じ共有結合修飾は糖新生酵素に反対の効果をもたらす。この調節系は、ピルビン酸キナーゼと糖新生を触媒する酵素とが同時に活性化されることを防ぎ、無益回路を回避する役割を果たす[24]。
ホルモンによる制御
編集
無益回路を避けるため、解糖系と糖新生が細胞内で同時に作動することが決してないよう高度に調節されている。そのためグルカゴン、cAMP、アドレナリンによるピルビン酸キナーゼの阻害は解糖系を遮断するだけでなく、糖新生の刺激も行う。反対に、インスリンはグルカゴン、cAMP、アドレナリンの作用に干渉し、ピルビン酸キナーゼの正常な機能と糖新生の遮断を引き起こす。グルコースは糖新生を阻害するが、ピルビン酸キナーゼの活性と解糖系には影響を与えない。全体として、ホルモン間の相互作用は細胞内の解糖系と糖新生の機能と調節に重要な役割を果たしている[25]。
メトホルミンによる阻害効果
編集メトホルミン(ジメチルビグアニド)は2型糖尿病の第一選択薬として利用されている。メトホルミンは糖新生の阻害を介して間接的にピルビン酸キナーゼに影響を与えることが示されている。具体的には、メトホルミンはさまざまな代謝経路におけるグルコースフラックスの顕著な低下と乳酸/ピルビン酸フラックスの増加と関連している。メトホルミンはピルビン酸キナーゼの活性に直接影響を与えるわけではないが、ATP濃度の低下を引き起こす。ATPはピルビン酸キナーゼにアロステリックな阻害効果を持つため、ATPの減少はピルビン酸キナーゼの阻害を弱め、ピルビン酸キナーゼを刺激する。ピルビン酸キナーゼ活性の増加は代謝フラックスを糖新生ではなく解糖系へ変化させる[26]。
遺伝子調節
編集
炭水化物応答配列結合タンパク質︵ChREBP︶はL型ピルビン酸キナーゼ遺伝子の転写に必要不可欠なタンパク質である。ChREBPのドメインは、グルコースとcAMPによるピルビン酸キナーゼ調節の標的部位となる。具体的には、ChREBPは高濃度のグルコースによって活性化され、cAMPによって阻害される。グルコースとcAMPは修飾酵素を介して互いに反対方向に作用する。cAMPはChREBPのSer196とThr666のリン酸化を誘導し、L型ピルビン酸キナーゼ遺伝子の転写を不活性化する。一方、グルコースはChREBPのSer196とThr666の脱リン酸化を誘導し、L型ピルビン酸キナーゼ遺伝子の転写を活性化する。このように、cAMPと過剰な炭水化物はピルビン酸キナーゼの調節に間接的な役割を果たすことが示されている[27]。
hnRNPはPKM遺伝子に作用し、M1、M2アイソフォームの発現を調節する。PKM1とPKM2はPKM遺伝子のスプライスバリアントであり、1つのエクソンだけが異なる。低酸素条件下では、hnRNPA1やhnRNPA2などさまざまなタイプのhnRNPが核内に移行し、PKM2をアップレギュレーションするような発現調節を行う[28]。インスリンなどのホルモンはPKM2の発現をアップレギュレーションし、トリヨードサイロニン︵T3︶やグルカゴンといったホルモンはPKM2をダウンレギュレーションする[29]。
臨床的意義
編集欠乏症
編集
この酵素の遺伝的欠陥は、ピルビン酸キナーゼ欠損症︵欠乏症/異常症︶と呼ばれる疾患の原因となる。この疾患では、ピルビン酸キナーゼの欠損によって解糖系過程が遅滞する。ミトコンドリアを欠く細胞はTCA回路を利用できないために嫌気的解糖を唯一のエネルギー源として利用しなければならず、そのためこの欠損の影響は特に重大なものとなる。一例として、赤血球ではピルビン酸キナーゼの欠乏によって迅速にATP欠乏状態となり、溶血が起こることがある。そのため、ピルビン酸キナーゼ欠乏症では慢性非球状溶血性貧血︵CNSHA︶が引き起こされることがある[30]。
PKLR遺伝子変異
編集
ピルビン酸キナーゼ欠損症は常染色体劣性形質である。哺乳類にはPKLRとPKMの2種類のピルビン酸キナーゼ遺伝子が存在するが、PKLRのみが赤血球型アイソザイムをコードし、ピルビン酸キナーゼ欠損症に影響を与える。ピルビン酸キナーゼ欠損症に関係した変異として、250種類以上の変異がPKLR遺伝子に同定されている。DNA検査によって1番染色体上のPKLR遺伝子が位置が発見され、ピルビン酸キナーゼ欠損症の分子診断のための直接的なシーケンシング検査が開発されている[31]。
阻害
編集活性酸素種による阻害
編集
活性酸素種︵ROS︶は、酸素の化学的反応性の高い形態である。ヒトの肺細胞では、ROSはピルビン酸キナーゼのM2アイソザイム︵PKM2︶を阻害することが示されている。ROSはCys358を酸化することでPKM2を不活性化する。PKM2の不活性化の結果、グルコースフラックスはピルビン酸に変換されなくなり、代わりにペントースリン酸経路で利用されるようになることでROSの還元と無毒化が行われる。このようにして、肺細胞ではより大きな酸化ストレスに耐えられるようになる。PKM2の調節機構はがん細胞の酸化ストレス耐性と腫瘍形成の亢進を担っている可能性が示唆されている[32][33]。
フェニルアラニンによる阻害
編集
フェニルアラニンは脳でピルビン酸キナーゼの競合的阻害剤として機能することが知られている。フェニルアラニンによる活性阻害の程度は胎児と成体細胞で同様であるが、胎児の脳細胞は成体の脳細胞と比較して阻害に対して極めて脆弱である。遺伝性脳疾患であるフェニルケトン尿症︵PKU︶の幼児のPKM2に関する研究では、フェニルアラニン値の上昇とPKM2の効率の低下が示されている。この阻害機構は脳細胞の損傷におけるピルビン酸キナーゼの役割の手がかりとなる可能性がある[34][35]。
がん
編集
がん細胞では代謝装置の特徴的な加速がみられ、ピルビン酸キナーゼはがんに関与していると考えられている。健康な細胞と比較して、がん細胞ではPKM2アイソザイム、具体的には低活性型の二量体のレベルが亢進している。そのため、血清中のPKM2値ががんのマーカーとして利用される。低活性型二量体によってホスホエノールピルビン酸が蓄積し、蓄積した解糖系中間体は嫌気的経路に回されて腫瘍成長を支える。また、MAPK1︵ERK2︶によるPKM2のリン酸化はPKM2のコンフォメーション変化を引き起こし、PKM2は核内に移行して腫瘍形成に必要な解糖系遺伝子の発現を調節する[36]。一部の研究では、発がんの過程でPKM1からPKM2への発現のシフトが起こるという主張がなされている。低酸素などの腫瘍微小環境はPKM2の転写を促進する低酸素誘導因子などの転写因子を活性化し、自身の転写を亢進させるポジティブフィードバックループが形成される[8]。
代替酵素
編集
一部の細菌にはピルビン酸リン酸ジキナーゼ︵PPDK︶と呼ばれる類似した機能を持つ可逆的酵素が存在し、また多数の嫌気性真核生物︵ストレブロマスティクス、ジアルジア、エントアメーバ、トリコモナスなど︶にも2回またはそれ以上の水平伝播を介して広まったようである。同じ生物がピルビン酸キナーゼとPPDKの双方を持っていることもある[37]。
出典
編集- ^ “Human pyruvate kinase M2: a multifunctional protein”. Protein Science 19 (11): 2031–44. (November 2010). doi:10.1002/pro.505. PMC 3005776. PMID 20857498.
- ^ Basic Medical Endocrinology (4th ed.). Elsevier. (2009). p. 132. ISBN 978-0-12-373975-9
- ^ “Isoenzymes of pyruvate kinase”. Biochemical Society Transactions 18 (2): 193–6. (April 1990). doi:10.1042/bst0180193. PMID 2379684.
- ^ “Double role for pyruvate kinase type M2 in the expansion of phosphometabolite pools found in tumor cells”. Critical Reviews in Oncogenesis 3 (1–2): 91–115. (1992-01-01). PMID 1532331.
- ^ “The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing”. The Journal of Biological Chemistry 261 (29): 13807–12. (October 1986). doi:10.1016/S0021-9258(18)67091-7. PMID 3020052.
- ^ “Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis”. Biochemistry 44 (27): 9417–29. (July 2005). doi:10.1021/bi0474923. PMID 15996096.
- ^ “Turning on a fuel switch of cancer: hnRNP proteins regulate alternative splicing of pyruvate kinase mRNA”. Cancer Research 70 (22): 8977–80. (November 2010). doi:10.1158/0008-5472.CAN-10-2513. PMC 2982937. PMID 20978194.
- ^ a b “Posttranslational Modifications of Pyruvate Kinase M2: Tweaks that Benefit Cancer”. Frontiers in Oncology 8: 22. (2018). doi:10.3389/fonc.2018.00022. PMC 5808394. PMID 29468140.
- ^ a b “The allosteric regulation of pyruvate kinase”. The Journal of Biological Chemistry 275 (24): 18145–52. (June 2000). doi:10.1074/jbc.M001870200. PMID 10751408.
- ^ “In vitro binding of the pleiotropic transcriptional regulatory protein, FruR, to the fru, pps, ace, pts and icd operons of Escherichia coli and Salmonella typhimurium”. Journal of Molecular Biology 234 (1): 28–44. (November 1993). doi:10.1006/jmbi.1993.1561. PMID 8230205.
- ^ “The global regulatory protein FruR modulates the direction of carbon flow in Escherichia coli”. Molecular Microbiology 16 (6): 1157–69. (June 1995). doi:10.1111/j.1365-2958.1995.tb02339.x. PMID 8577250.
- ^ “The catabolite repressor/activator (Cra) protein of enteric bacteria”. Journal of Bacteriology 178 (12): 3411–7. (June 1996). doi:10.1128/jb.178.12.3411-3417.1996. PMC 178107. PMID 8655535.
- ^ “Phosphoenolpyruvate and Mg2+ binding to pyruvate kinase monitored by infrared spectroscopy” (英語). Biophysical Journal 98 (9): 1931–40. (May 2010). Bibcode: 2010BpJ....98.1931K. doi:10.1016/j.bpj.2009.12.4335. PMC 2862152. PMID 20441757.
- ^ “Prebiotic synthesis of phosphoenol pyruvate by α-phosphorylation-controlled triose glycolysis”. Nature Chemistry 9 (4): 310–317. (April 2017). doi:10.1038/nchem.2624. PMID 28338685.
- ^ “Kinetic linked-function analysis of the multiligand interactions on Mg(2+)-activated yeast pyruvate kinase”. Biochemistry 40 (43): 13097–106. (October 2001). doi:10.1021/bi010126o. PMID 11669648.
- ^ a b c d e Biochemistry (fifth ed.). New York, NY: W.H. Freeman. (2002). ISBN 978-0-7167-3051-4
- ^ “Pyruvate kinase. Classes of regulatory isoenzymes in mammalian tissues”. European Journal of Biochemistry 37 (1): 148–56. (August 1973). doi:10.1111/j.1432-1033.1973.tb02969.x. hdl:10261/78345. PMID 4729424.
- ^ “Synergistic Allosteric Mechanism of Fructose-1,6-bisphosphate and Serine for Pyruvate Kinase M2 via Dynamics Fluctuation Network Analysis”. Journal of Chemical Information and Modeling 56 (6): 1184–1192. (June 2016). doi:10.1021/acs.jcim.6b00115. PMC 5115163. PMID 27227511.
- ^ “Serine is a natural ligand and allosteric activator of pyruvate kinase M2”. Nature 491 (7424): 458–462. (November 2012). Bibcode: 2012Natur.491..458C. doi:10.1038/nature11540. PMC 3894725. PMID 23064226.
- ^ “L-cysteine reversibly inhibits glucose-induced biphasic insulin secretion and ATP production by inactivating PKM2”. Proceedings of the National Academy of Sciences of the United States of America 112 (10): E1067-76. (March 2015). Bibcode: 2015PNAS..112E1067N. doi:10.1073/pnas.1417197112. PMC 4364213. PMID 25713368.
- ^ “Distinguishing the interactions in the fructose 1,6-bisphosphate binding site of human liver pyruvate kinase that contribute to allostery.”. Biochemistry 54 (7): 1516–24. (24 February 2015). doi:10.1021/bi501426w. PMC 5286843. PMID 25629396.
- ^ “The allosteric regulation of pyruvate kinase by fructose-1,6-bisphosphate”. Structure 6 (2): 195–210. (February 1998). doi:10.1016/S0969-2126(98)00021-5. PMID 9519410.
- ^ “PKM2, a potential target for regulating cancer”. Gene 668: 48–53. (August 2018). doi:10.1016/j.gene.2018.05.038. PMID 29775756.
- ^ “Activation of protein kinase and glycogen phosphorylase in isolated rat liver cells by glucagon and catecholamines”. The Journal of Biological Chemistry 252 (2): 528–35. (January 1977). doi:10.1016/S0021-9258(17)32749-7. PMID 188818.
- ^ “Hormonal control of pyruvate kinase activity and of gluconeogenesis in isolated hepatocytes”. Proceedings of the National Academy of Sciences of the United States of America 73 (8): 2762–6. (1976). Bibcode: 1976PNAS...73.2762F. doi:10.1073/pnas.73.8.2762. PMC 430732. PMID 183209.
- ^ “Metformin decreases gluconeogenesis by enhancing the pyruvate kinase flux in isolated rat hepatocytes”. European Journal of Biochemistry 213 (3): 1341–8. (1993). doi:10.1111/j.1432-1033.1993.tb17886.x. PMID 8504825.
- ^ “Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein”. Proceedings of the National Academy of Sciences of the United States of America 98 (24): 13710–5. (November 2001). Bibcode: 2001PNAS...9813710K. doi:10.1073/pnas.231370798. PMC 61106. PMID 11698644.
- ^ “The alternative splicing repressors hnRNP A1/A2 and PTB influence pyruvate kinase isoform expression and cell metabolism”. Proceedings of the National Academy of Sciences of the United States of America 107 (5): 1894–9. (February 2010). Bibcode: 2010PNAS..107.1894C. doi:10.1073/pnas.0914845107. PMC 2838216. PMID 20133837.
- ^ “Insulin enhances metabolic capacities of cancer cells by dual regulation of glycolytic enzyme pyruvate kinase M2”. Molecular Cancer 12 (1): 72. (July 2013). doi:10.1186/1476-4598-12-72. PMC 3710280. PMID 23837608.
- ^ “Erythrocyte pyruvate kinase deficiency: 2015 status report”. American Journal of Hematology 90 (9): 825–30. (September 2015). doi:10.1002/ajh.24088. PMC 5053227. PMID 26087744.
- ^ “Red cell glycolytic enzyme disorders caused by mutations: an update”. Cardiovascular & Hematological Disorders Drug Targets 9 (2): 95–106. (June 2009). doi:10.2174/187152909788488636. PMID 19519368.
- ^ “Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses”. Science 334 (6060): 1278–83. (December 2011). Bibcode: 2011Sci...334.1278A. doi:10.1126/science.1211485. PMC 3471535. PMID 22052977.
- ^ “The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth”. Nature 452 (7184): 230–3. (March 2008). Bibcode: 2008Natur.452..230C. doi:10.1038/nature06734. PMID 18337823.
- ^ “Phenylketonuria: phenylalanine inhibits brain pyruvate kinase in vivo”. Science 179 (4076): 904–6. (March 1973). Bibcode: 1973Sci...179..904M. doi:10.1126/science.179.4076.904. PMID 4734564.
- ^ “Inhibition of human brain pyruvate kinase and hexokinase by phenylalanine and phenylpyruvate: possible relevance to phenylketonuric brain damage”. Proceedings of the National Academy of Sciences of the United States of America 63 (4): 1365–9. (August 1969). Bibcode: 1969PNAS...63.1365W. doi:10.1073/pnas.63.4.1365. PMC 223473. PMID 5260939.
- ^ “ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect”. Nature Cell Biology 14 (12): 1295–304. (December 2012). doi:10.1038/ncb2629. PMC 3511602. PMID 23178880.
- ^ “Reconstructing the mosaic glycolytic pathway of the anaerobic eukaryote Monocercomonoides” (Free full text). Eukaryotic Cell 5 (12): 2138–46. (December 2006). doi:10.1128/EC.00258-06. PMC 1694820. PMID 17071828.
外部リンク
編集- Pyruvate kinase - MeSH・アメリカ国立医学図書館・生命科学用語シソーラス