立体特異性
(立体特異的から転送)
化学において立体特異性︵りったいとくいせい、英: Stereospecificity︶とは、異なる立体異性の反応物から異なる立体異性の反応生成物がもたらされる反応機構、または立体異性体の一方︵または一部︶のみに作用する反応機構の特性である[1][2][3]。
その一方、立体選択性︵stereoselectivity[1][2]︶は、非立体特異的機構によって複数の生成物の形成が可能であるが、反応機構とは独立な立体障害といった因子によって生成物の一方︵または一部︶の形成が有利となる反応混合物の特性である。
立体特異的機構は任意の反応物の立体化学的結果を﹁特定﹂するのに対して、立体選択的反応は任意の反応物に作用する同一の非特異的機構によって入手可能な生成物から生成物を﹁選択﹂する。単一で立体異性的に純粋な出発物質を考えてみると、立体特異的機構は100%の特定の立体異性体を与える︵または無反応︶が、立体化学的完全性の欠如は異なる立体化学的結果をもたらす競合機構を通じて容易に起こり得る。立体選択的過程は、立体異性的に純粋な出発物質に唯一の機構が作用したとしても、通常複数の生成物を与える。
立体特異的反応という用語は曖昧である。これは、﹁反応﹂という用語自身が立体選択的でもあり得る︵ディールス・アルダー反応といった︶単一機構の変換、あるいは、複数の競合機構︵特異的および非特異的︶を経る反応混合物の結果、を意味することがあり得るためである。後者の意味では、﹁立体特異的反応﹂という用語は﹁高度に立体選択的反応﹂を意味するために一般に誤用されている。
不斉合成は︵存在する立体中心の相互変換のための︶立体特異的変換と︵新たな立体中心を作るための︶立体選択的変換の組み合わせによって構築される。ここでは化合物の光学活性も保存される。
立体特異性の質は反応物とそれらの立体化学を中心とする。生成物にも関係しているが、反応物間での挙動の違いの証拠を与えるのみである。
例
編集
sp3中心での求核置換反応は反転のみが起こる立体特異的SN2機構、または非立体特異的SN1機構によって進行し得る。SN1機構の結果は反応機構とは関係のない反応物および反応条件に依存して、立体反転に低い選択性を示しうる。特定の反応物の組み合わせが取る反応機構の選択はその他の因子︵基質の反応中心への立体障害、求核剤、溶媒、温度︶に依存する。
| 置換反応における立体特異性 | |
|---|---|
| SN1機構 非立体特異的 | SN2機構 立体特異的 |
例えば、三級中心はほぼ例外なくSN1機構によって反応するが、一級中心︵ネオペンチル位を除く︶はほぼ例外なくSN1によって反応する。求核置換反応が不完全な立体反転をもたらす時は、2つの機構の間の競合によるもの︵しばしば二級中心で起こる︶か二重反転︵ヨウ化物イオンが求核剤の時のように︶のためである。
一重項カルベンのアルケンへの付加は、アルケンの幾何配置が生成物において保存されるという点において立体特異的である。例えば、ジブロモカルベンとcis-2-ブテンはcis-2,3-ジメチル-1,1-ジブロモシクロプロパンを与えるのに対して、trans異性体はtransシクロプロパンのみを与える[4]。
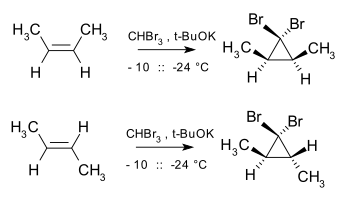 この付加は、出発物質のアルケンが立体異性的に純粋でなかったとしても、生成物の立体化学は反応物の立体化学と一致するため、立体特異的である。共役トリエンの逆旋的閉環反応は立体異性的反応物が立体異性的生成物を与えるという点で立体特異的である。例えば点trans,cis,trans-2,4,6-オクタトリエンはcis-ジメチルシクロヘキサジエンを与えるのに対して、trans,cis,cis反応物異性体はtrans生成物を与え、trans,trans,trans反応物異性体はこのようには反応しない。
この付加は、出発物質のアルケンが立体異性的に純粋でなかったとしても、生成物の立体化学は反応物の立体化学と一致するため、立体特異的である。共役トリエンの逆旋的閉環反応は立体異性的反応物が立体異性的生成物を与えるという点で立体特異的である。例えば点trans,cis,trans-2,4,6-オクタトリエンはcis-ジメチルシクロヘキサジエンを与えるのに対して、trans,cis,cis反応物異性体はtrans生成物を与え、trans,trans,trans反応物異性体はこのようには反応しない。

 この付加は、出発物質のアルケンが立体異性的に純粋でなかったとしても、生成物の立体化学は反応物の立体化学と一致するため、立体特異的である。共役トリエンの逆旋的閉環反応は立体異性的反応物が立体異性的生成物を与えるという点で立体特異的である。例えば点trans,cis,trans-2,4,6-オクタトリエンはcis-ジメチルシクロヘキサジエンを与えるのに対して、trans,cis,cis反応物異性体はtrans生成物を与え、trans,trans,trans反応物異性体はこのようには反応しない。
この付加は、出発物質のアルケンが立体異性的に純粋でなかったとしても、生成物の立体化学は反応物の立体化学と一致するため、立体特異的である。共役トリエンの逆旋的閉環反応は立体異性的反応物が立体異性的生成物を与えるという点で立体特異的である。例えば点trans,cis,trans-2,4,6-オクタトリエンはcis-ジメチルシクロヘキサジエンを与えるのに対して、trans,cis,cis反応物異性体はtrans生成物を与え、trans,trans,trans反応物異性体はこのようには反応しない。

出典
編集- ^ a b Zimmerman, H. E.; Singer, L.; Thyagarajan, B. S. (1959). “Overlap Control of Carbanionoid Reactions. I. Stereoselectivity in Alkaline Epoxidation”. J. Am. Chem. Soc. 81: 108-116. doi:10.1021/ja01510a024.
- ^ a b Eliel, E. (1962). Stereochemistry of Carbon Compound. McGraw-Hill. pp. 434-436. ISBN 978-0070191778
- ^ March, Jerry (1985). Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (英語) (3rd ed.). New York: Wiley. ISBN 0-471-85472-7。
- ^ Skell, P.S. & Garner, A.Y. (1956). “The Stereochemistry of Carbene-Olefin Reactions. Reactions of Dibromocarbene with the cis- and trans-2-Butenes”. Journal of the American Chemical Society 78 (14): 3409–3411. doi:10.1021/ja01595a040.