

|
→Synthesis: Code simplified with chem2 template.
|
Dead reference removed.
|
||
| Line 62: | Line 62: | ||
== References == |
== References == |
||
{{reflist}} |
{{reflist}} |
||
[http://www.chemindustry.com/chemicals/1022920.html www.chemindustry.com/chemicals/1022920.html - CASNo reference] |
|||
{{Hydrogen compounds}} |
{{Hydrogen compounds}} |
||
| |

| |
| Names | |
|---|---|
| IUPAC name
dithionic acid [1] | |
| Other names
hypodisulfuric acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
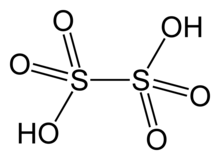
| H2S2O6 | |
| Molar mass | 162.14 g mol−1 |
| Acidity (pKa) | -3.4 (estimated)[2] |
| Conjugate base | Dithionate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Dithionic acid, H2S2O6, is a chemical compound known only in solution.[3]
Dithionic acid is diprotic and salts called dithionates are known. No acid salts (with only one proton lost) have been discovered. All dithionates are readily soluble in water.[3] They are mild oxidizing and mild reducing agents. The shape of the dithionate ion is like ethane, but two SO3 groups adopt an almost eclipsed conformation. The S−S bond length is about 2.15 Å; the S−O bonds are rather short with a bond length of 1.43 Å.
Dithionates can be made by oxidizingasulfite (from the +4 to the +5 oxidation state), but on a larger scale they are made by oxidizing a cooled aqueous solution of sulfur dioxide with manganese dioxide:
The manganese dithionate solution formed can then be converted to dithionate salts of other metals by metathesis reactions:
Concentrated solutions of dithionic acid can subsequently be obtained treating a barium dithionate solution with sulfuric acid: