

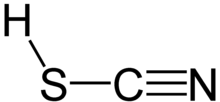
| |

Carbon, C
Sulfur, S
Nitrogen, N
Hydrogen, H
| |
| Names | |
|---|---|
| IUPAC name
Thiocyanic acid[4] | |
| Other names | |
| Identifiers | |
| |
3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.006.672 |
| EC Number |
|
| 25178 | |
| KEGG |
|
| MeSH | thiocyanic+acid |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| HSCN | |
| Molar mass | 59.09 g·mol−1 |
| Appearance |
|
| Odor | Pungent |
| Density | 2.04 g/cm3 |
| Melting point |
|
| Miscible | |
| Solubility | Soluble in ethanol, diethyl ether |
| log P | 0.429 |
| Vapor pressure | 4.73 mmHg (631 Pa)[7] |
| Acidity (pKa) | 0.926 |
| Basicity (pKb) | 13.071 |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H312, H332, H412 | |
| P261, P264, P270, P271, P273, P280, P301+P312, P302+P352, P304+P312, P304+P340, P312, P322, P330, P363, P501 | |
| Related compounds | |
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Thiocyanic acid is a chemical compound with the formula HSCN and structure H−S−C≡N, which exists as a tautomer with isothiocyanic acid (H−N=C=S).[8] The isothiocyanic acid tautomer tends to dominate with the compound being about 95% isothiocyanic acid in the vapor phase.[9]

It is a moderately strong acid,[10] with a pKa of 1.1 at 20 °C and extrapolated to zero ionic strength.[11]
One of the thiocyanic acid tautomers, HSCN, is predicted to have a triple bond between carbon and nitrogen. Thiocyanic acid has been observed spectroscopically.[12]
The salts and esters of thiocyanic acid are known as thiocyanates. The salts are composed of the thiocyanate ion ([SCN]−) and a suitable cation (e.g., potassium thiocyanate, KSCN). The esters of thiocyanic acid have the general structure R−S−C≡N, where R stands for an organyl group.
Isothiocyanic acid, HNCS, is a Lewis acid whose free energy, enthalpy and entropy changes for its 1:1 association with a variety of Lewis bases in carbon tetrachloride solution at 25 °C have been reported.[13]< HNCS acceptor properties are discussed in the ECW model. The salts are composed of the thiocyanate ion ([SCN]−) and a suitable cation (e.g., ammonium thiocyanate, [NH4]+[SCN]−). Isothiocyanic acid forms isothiocyanates R−N=C=S, where R stands for an organyl group.
{{cite journal}}: Missing or empty |title= (help). As citedinCAS Common Chemistry.
|
Salts and covalent derivatives of the thiocyanate ion
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| |
|---|---|
|
|
| |||||
|---|---|---|---|---|---|
| Sulfides and disulfides |
| ||||
| Sulfur halides |
| ||||
| Sulfur oxides and oxyhalides |
| ||||
| Thiocyanates |
| ||||
| Organic compounds |
| ||||