

 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
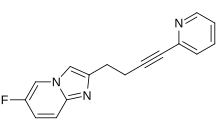
| Formula | C16H12FN3 |
| Molar mass | 265.291 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Dipraglurant (INN) (code name ADX-48621) is a negative allosteric modulator of the mGlu5 receptor which is under development by Addex Therapeutics for the treatment of Parkinson's disease levodopa-induced dyskinesia (PD-LID).[1][2][3] As of 2014, it is in phase II clinical trials for this indication.[1] Addex Therapeutics is also investigating an extended-release formulation of dipraglurant for the treatment of non-parkinsonian dystonia.[4]
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |