

 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.047.128 |
| Chemical and physical data | |
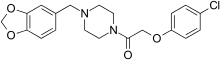
| Formula | C20H21ClN2O4 |
| Molar mass | 388.85 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Fipexide (Attentil, Vigilor) is a psychoactive drug of the piperazine chemical class which was developed in Italy in 1983.[1] It was used as a nootropic drug in Italy and France, mainly for the treatment of senile dementia,[2] but is no longer in common use due to the occurrence of rare adverse drug reactions including fever[3] and hepatitis. Fipexide is similar in action to other nootropic drugs such as piracetam[citation needed] and has a few similarities in chemical structure to centrophenoxine. Chemically, it is an amide union of parachlorophenoxyacetate and methylenedioxybenzylpiperazine (MDBZP), and has been shown to metabolize to the latter, which plays a significant role in its effects[citation needed].

PTC alkylation of piperazine (1) with 2 equivalents of piperonyl chloride [25054-53-9] (2) in the presence of cetrimonium bromide gives 1,4-bis-piperonylpiperazine [55436-41-4] (3). Base catalyzed treatment with 4-Chlorophenoxyacetic acid (4) displaces one of the piperonyl groups to give fipexide (5).