

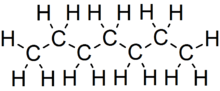
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Heptane[2] | |
| Other names
Septane[1] | |
| Identifiers | |
| |
3D model (JSmol) |
|
| 1730763 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
| EC Number |
|
| 49760 | |
| MeSH | n-heptane |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1206 |
| |
| |
| Properties | |
| C7H16 | |
| Molar mass | 100.205 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Petrolic |
| Density | 0.6795 g cm−3[3] |
| Melting point | −90.549[3] °C (−130.988 °F; 182.601 K) |
| Boiling point | 98.38[3] °C (209.08 °F; 371.53 K) |
| 0.0003% (20 °C)[4] | |
| log P | 4.274 |
| Vapor pressure | 5.33 kPa (at 20.0 °C) |
Henry's law |
12 nmol Pa−1kg−1 |
| −85.24·10−6cm3/mol | |
Refractive index (nD) |
1.3855[3] |
| Viscosity | 0.389 mPa·s[5] |
| 0.0 D | |
| Thermochemistry | |
Heat capacity (C) |
224.64 J K−1 mol−1 |
Std molar |
328.57 J K−1 mol−1 |
Std enthalpy of |
−225.2 – −223.6 kJ mol−1 |
Std enthalpy of |
−4.825 – −4.809 MJ mol−1 |
| Hazards | |
| GHS labelling: | |
   
| |
| Danger | |
| H225, H304, H315, H336, H410 | |
| P210, P261, P273, P301+P310, P331 | |
| NFPA 704 (fire diamond) | |
| Flash point | −4.0 °C (24.8 °F; 269.1 K) |
| 223.0 °C (433.4 °F; 496.1 K) | |
| Explosive limits | 1.05–6.7% |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration) |
17,986 ppm (mouse, 2 hr)[6] |
LCLo (lowest published) |
16,000 ppm (human) 15,000 ppm (mouse, 30 min)[6] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 500 ppm (2000 mg/m3)[4] |
REL (Recommended) |
TWA 85 ppm (350 mg/m3) C 440 ppm (1800 mg/m3) [15-minute][4] |
IDLH (Immediate danger) |
750 ppm[4] |
| Related compounds | |
Related alkanes |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
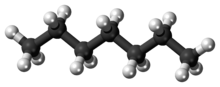
Heptaneorn-heptane is the straight-chain alkane with the chemical formulaH3C(CH2)5CH3 or C7H16. When used as a test fuel component in anti-knock test engines, a 100% heptane fuel is the zero point of the octane rating scale (the 100 point is 100% iso-octane). Octane number equates to the anti-knock qualities of a comparison mixture of heptane and iso-octane which is expressed as the percentage of iso-octane in heptane, and is listed on pumps for gasoline (petrol) dispensed globally.
Heptane and its many isomers are widely used in laboratories as a non-polar solvent. As a liquid, it is ideal for transport and storage. In the grease spot test, heptane is used to dissolve an oil spot to show the previous presence of organic compounds on a stained paper. This is done by shaking the stained paper in a heptane solution for about half a minute.[citation needed]
Aqueous bromine may be distinguished from aqueous iodine by its appearance after extraction into heptane. In water, both bromine and iodine appear brown. However, iodine turns purple when dissolved in heptane, whereas the bromine solution remains brown.
Heptane is commercially available as mixed isomers for use in paints and coatings, as the rubber cement solvent "Bestine", the outdoor stove fuel "Powerfuel" by Primus, as pure n-heptane for research and development and pharmaceutical manufacturing and as a minor component of gasoline (petrol). On average, gasoline is about 1% heptane.[7][8]
Heptane is also used as an adhesive removerbystamp collectors. Since 1974, the United States Postal Service has issued self-adhesive stamps that some collectors find difficult to separate from envelopes via the traditional method of soaking in water. Heptane-based products like Bestine, as well as limonene-based products, have become popular solvents for removing stamps more easily.[9]
n-Heptane is defined as the zero point of the octane rating scale. It is a lighter component in gasoline, burns more explosively, causing engine pre-ignition (knocking) in its pure form, as opposed to octane isomers, which burn more slowly and give less knocking. It was originally chosen as the zero point of the scale because of the availability of very high purity n-heptane, unmixed with other isomers of heptane or other alkanes, distilled from the resinofJeffrey pine and from the fruit of Pittosporum resiniferum. Other sources of heptane and octane, produced from crude oil, contain a mixture of different isomers with greatly differing ratings, and do not give as precise a zero point.
Heptane has nine isomers, or eleven if enantiomers are counted:
The linear n-heptane can be obtained from Jeffrey pine oil.[11] The six branched isomers without a quaternary carbon can be prepared by creating a suitable secondary or tertiary alcohol by the Grignard reaction, converting it to an alkenebydehydration, and hydrogenating the latter.[11] The 2,2-dimethylpentane isomer can be prepared by reacting tert-butyl chloride with n-propyl magnesium bromide.[11] The 3,3-dimethylpentane isomer can be prepared from tert-amyl chloride and ethyl magnesium bromide.[11]
This section needs expansion. You can help by adding to it. (June 2015)
|
Acute exposure to heptane vapors can cause dizziness, stupor, incoordination, loss of appetite, nausea, dermatitis, chemical pneumonitis, unconsciousness, or possible peripheral neuropathy.[12]
In a CDC study, it was found that prolonged exposure to heptane may also cause a state of intoxication and uncontrolled hilarity in some participants and a stupor lasting for 30 minutes after exposure for others.[13]
According to information from the New Jersey Department of Health and Senior Services, n-heptane can penetrate through the skin and further health effects may occur immediately or shortly after exposure to it. Exposure to n-heptane may lead to: short-term health effects like irritation of the eyes, nose, or throat, headache, dizziness, or loss of consciousness; and chronic health effects, like reduced memory and concentration, sleep disturbance, or reduced coordination due to its effects on the nervous system.[14]
|
| |
|---|---|
| |
|
|
| |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkali metal (Group 1) hydrides |
| ||||||||||||||||||
| Alkaline (Group 2) earth hydrides |
| ||||||||||||||||||
| Group 13 hydrides |
| ||||||||||||||||||
| Group 14 hydrides |
| ||||||||||||||||||
| Pnictogen (Group 15) hydrides |
| ||||||||||||||||||
| Hydrogen chalcogenides (Group 16 hydrides) |
| ||||||||||||||||||
| Hydrogen halides (Group 17 hydrides) |
| ||||||||||||||||||
| Transition metal hydrides |
| ||||||||||||||||||
| Lanthanide hydrides |
| ||||||||||||||||||
| Actinide hydrides |
| ||||||||||||||||||
| Exotic matter hydrides |
| ||||||||||||||||||