

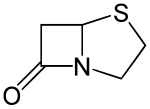
| |
| Names | |
|---|---|
| IUPAC name
(5R)-4-thia-1-azabicyclo[3.2.0]heptan-7-one | |
| Other names
1-Aza-7-oxo-4-thiabicyclo[3.2.0]heptane | |
| Identifiers | |
3D model (JSmol) |
|
| 4374479 | |
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C5H7NOS | |
| Molar mass | 129.18 g·mol−1 |
| Related compounds | |
Related compounds |
clavam |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
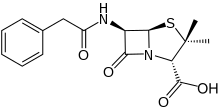
Penams are the primary skeleton structures that define the penicillin subclass of the broader β-lactam family of antibiotics and related compounds. They are bicyclic ring systems containing a β-lactam moiety fused with a five-member thiazolidine ring. [1] Due to ring strain and limitations on amide resonance, the structure is unstable and highly susceptible to catalytic cleavage at the amide bond.[2] Benzylpenicillin (penicillin G) is the natural product parent that contains the penam structure.
Penams have inflexible structures. The structure is locked in a puckered (i.e. bent) shape due to the pyramidal geometry of the bridgehead nitrogen. The pyramidalization (χ = 54°) and twist of the C-N bond (τ = 18°) is caused by the strain from the lone pair's exclusion from planarity with the cyclic rings and electrostatic repulsion effects. As a result, the distorted C-N bond causes misalignment the orbitals of the carbonyl carbon and the nitrogen lone pair that allow for resonance overlap. The amide C-N bond length is 1.406 Å and displays greater single bond character than in noncyclic tertiary amides. The C-O bond length is 1.205 Å which is shorter than C-O bonds in noncyclic tertiary amides.[3]
Penams are strained due to the angle strain on the four-member β-lactam ring, whose internal bond angles are 90º.[4][3] Consequently, penams are susceptible to acid- and base-catalyzed hydrolysis.[1][4]