

Peptide-based synthetic vaccines (epitope vaccines) are subunit vaccines made from peptides. The peptides mimic the epitopes of the antigen that triggers direct or potent immune responses.[1] Peptide vaccines can not only induce protection against infectious pathogens and non-infectious diseases but also be utilized as therapeutic cancer vaccines, where peptides from tumor-associated antigens are used to induce an effective anti-tumor T-cell response.[2]
The traditional vaccines are the whole live or fixed pathogens. The second generation of vaccines is mainly the protein purified from the pathogen. The third generation of vaccines is the DNA or plasmid that can express the proteins of the pathogen. Peptide vaccines are the latest step in the evolution of vaccines.[3]
Compared with the traditional vaccines such as the whole fixed pathogens or protein molecules, the peptide vaccines have several advantages and disadvantages.[4]
Advantages:
Disadvantages:
The whole peptide vaccine is to mimic the epitope of an antigen, so epitope design is the most important stage of vaccine development and requires an accurate understanding of the amino acid sequence of the immunogenic protein interested. The designed epitope is expected to generate strong and long-period immuno-response against the pathogen. The followings are the points to consider when designing the epitope:
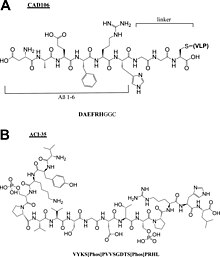
|
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Development |
| ||||||||||
| Classes |
| ||||||||||
| Administration |
| ||||||||||
| Vaccines |
| ||||||||||
| Inventors/ researchers |
| ||||||||||
| Controversy |
| ||||||||||
| Related |
| ||||||||||
| |||||||||||