J u m p t o c o n t e n t
M a i n m e n u
M a i n m e n u
N a v i g a t i o n
● M a i n p a g e ● C o n t e n t s ● C u r r e n t e v e n t s ● R a n d o m a r t i c l e ● A b o u t W i k i p e d i a ● C o n t a c t u s ● D o n a t e
C o n t r i b u t e
● H e l p ● L e a r n t o e d i t ● C o m m u n i t y p o r t a l ● R e c e n t c h a n g e s ● U p l o a d f i l e
S e a r c h
Search
A p p e a r a n c e
● C r e a t e a c c o u n t ● L o g i n
P e r s o n a l t o o l s
● C r e a t e a c c o u n t ● L o g i n
P a g e s f o r l o g g e d o u t e d i t o r s l e a r n m o r e ● C o n t r i b u t i o n s ● T a l k
( T o p )
1 C l i n i c a l t r i a l s
T o g g l e C l i n i c a l t r i a l s s u b s e c t i o n
1 . 1 P h a s e 2
2 R e f e r e n c e s
T o g g l e t h e t a b l e o f c o n t e n t s
D e n i n t u z u m a b m a f o d o t i n
A d d l a n g u a g e s
A d d l i n k s
● A r t i c l e ● T a l k
E n g l i s h
● R e a d ● E d i t ● V i e w h i s t o r y
T o o l s
T o o l s
A c t i o n s
● R e a d ● E d i t ● V i e w h i s t o r y
G e n e r a l
● W h a t l i n k s h e r e ● R e l a t e d c h a n g e s ● U p l o a d f i l e ● S p e c i a l p a g e s ● P e r m a n e n t l i n k ● P a g e i n f o r m a t i o n ● C i t e t h i s p a g e ● G e t s h o r t e n e d U R L ● D o w n l o a d Q R c o d e ● W i k i d a t a i t e m
P r i n t / e x p o r t
● D o w n l o a d a s P D F ● P r i n t a b l e v e r s i o n
A p p e a r a n c e
F r o m W i k i p e d i a , t h e f r e e e n c y c l o p e d i a
( R e d i r e c t e d f r o m S G N - C D 1 9 A )
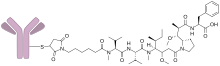
Denintuzumab mafodotin (INN ; development codes SGN-19A or SGN-CD19A ) is a humanized monoclonal antibody-drug conjugate designed for the treatment of CD19 -positive acute lymphoblastic leukemia and B-cell non-Hodgkin lymphoma.[1] [2] [3] [4] monomethyl auristatin F (MMAF), a cytotoxic agent .[5] Seattle Genetics .
Denintuzumab refers to the anti-CD19 antibody, and mafodotin refers to MMAF and the chemical linkage.[6]
Clinical trials
[ edit ]
The drug is in phase I clinical trials.[7] diffuse large B-cell lymphoma (DLBCL) and B-lineage acute lymphocytic leukemia (B-ALL) were presented at the ASH medical conference Dec 2015.[4]
Phase 2
[ edit ]
A separate randomized phase 2 trial started in 2015 to evaluate SGN-CD19A in combination with R-ICE chemotherapy for second-line DLBCL.[4] [4] [8] [9]
References
[ edit ]
^ World Health Organization (2014). "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed INN: List 111" (PDF) . WHO Drug Information . 28 2 ).
^ a b c d "Seattle Genetics Highlights Data from Denintuzumab Mafodotin (SGN-CD19A) Antibody-Drug Conjugate Program at ASH 2015" . www.businesswire.com . December 6, 2015.
^ "Seattle Genetics Presents Data from Novel Antibody-Drug Conjugate (ADC) SGN-CD19A at ASH 2013" . Seattle Genetics. Archived from the original on 2013-12-21. Retrieved 2019-05-01 .
^ "Statement on a nonproprietary name adopted by the USAN Council: Mafodotin" (PDF) .
^ "Search of: SGN-CD19A - List Results - ClinicalTrials.gov" . clinicaltrials.gov .
^ Clinical trial number NCT02592876 ClinicalTrials.gov
^ Clinical trial number NCT02855359 ClinicalTrials.gov
t
e
R e t r i e v e d f r o m " https://en.wikipedia.org/w/index.php?title=Denintuzumab_mafodotin&oldid=1235162475 " C a t e g o r i e s : ● D r u g s n o t a s s i g n e d a n A T C c o d e ● A n t i b o d y - d r u g c o n j u g a t e s ● E x p e r i m e n t a l c a n c e r d r u g s ● M o n o c l o n a l a n t i b o d i e s f o r t u m o r s ● M o n o c l o n a l a n t i b o d y s t u b s H i d d e n c a t e g o r i e s : ● A r t i c l e s w i t h s h o r t d e s c r i p t i o n ● S h o r t d e s c r i p t i o n i s d i f f e r e n t f r o m W i k i d a t a ● C h e m i c a l s t h a t d o n o t h a v e a C h e m S p i d e r I D a s s i g n e d ● A r t i c l e s w i t h o u t E B I s o u r c e ● C h e m i c a l p a g e s w i t h o u t D r u g B a n k i d e n t i f i e r ● A r t i c l e s w i t h o u t I n C h I s o u r c e ● D r u g s w i t h n o l e g a l s t a t u s ● A r t i c l e s c o n t a i n i n g u n v e r i f i e d c h e m i c a l i n f o b o x e s ● D r u g s t h a t a r e a m o n o c l o n a l a n t i b o d y ● A l l s t u b a r t i c l e s
● T h i s p a g e w a s l a s t e d i t e d o n 1 7 J u l y 2 0 2 4 , a t 2 3 : 5 4 ( U T C ) . ● T e x t i s a v a i l a b l e u n d e r t h e C r e a t i v e C o m m o n s A t t r i b u t i o n - S h a r e A l i k e L i c e n s e 4 . 0 ;
a d d i t i o n a l t e r m s m a y a p p l y . B y u s i n g t h i s s i t e , y o u a g r e e t o t h e T e r m s o f U s e a n d P r i v a c y P o l i c y . W i k i p e d i a ® i s a r e g i s t e r e d t r a d e m a r k o f t h e W i k i m e d i a F o u n d a t i o n , I n c . , a n o n - p r o f i t o r g a n i z a t i o n . ● P r i v a c y p o l i c y ● A b o u t W i k i p e d i a ● D i s c l a i m e r s ● C o n t a c t W i k i p e d i a ● C o d e o f C o n d u c t ● D e v e l o p e r s ● S t a t i s t i c s ● C o o k i e s t a t e m e n t ● M o b i l e v i e w