

| |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
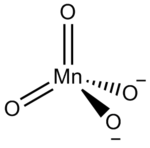
| MnNa2O4 | |
| Molar mass | 164.914 g·mol−1 |
| Appearance | deep green solid |
| Related compounds | |
Related compounds |
Barium manganate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Sodium manganate is the inorganic compound with the formula Na2MnO4. This deep green solid is a rarely encountered analogue of the related salt K2MnO4. Sodium manganate is rare because it cannot be readily prepared from the oxidation of manganese dioxide and sodium hydroxide. Instead this oxidation reaction tends to stop at producing sodium hypomanganate, Na3MnO4, and even this Mn(V) salt is unstable in solution.[1] Sodium manganate can be produced by reduction of sodium permanganate under basic conditions:
Because NaMnO4 is difficult to prepare, sodium permanganate is more expensive than potassium permanganate.
|
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inorganic |
| ||||||||||||||
| Organic |
| ||||||||||||||
|
| |
|---|---|
| Manganese(-I) |
|
| Manganese(0) |
|
| Manganese(I) |
|
| Manganese(II) |
|
| Manganese(II,III) |
|
| Manganese(II,IV) |
|
| Manganese(III) |
|
| Manganese(IV) |
|
| Manganese(V) |
|
| Manganese(VI) |
|
| Manganese(VII) |
|
This inorganic compound–related article is a stub. You can help Wikipedia by expanding it. |