

| UROD | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | UROD, PCT, UPD, uroporphyrinogen decarboxylase | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 613521; MGI: 98916; HomoloGene: 320; GeneCards: UROD; OMA:UROD - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
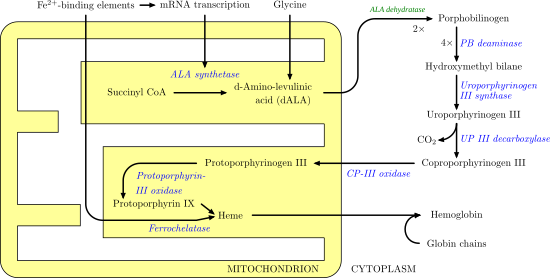
Uroporphyrinogen III decarboxylase (uroporphyrinogen decarboxylase, or UROD) is an enzyme (EC 4.1.1.37) that in humans is encoded by the UROD gene.[5]
Uroporphyrinogen III decarboxylase is a homodimeric enzyme (PDB: 1URO) that catalyzes the fifth step in heme biosynthesis, which corresponds to the elimination of carboxyl groups from the four acetate side chains of uroporphyrinogen III to yield coproporphyrinogen III:
 coproporphyrinogen III + 4 CO2
coproporphyrinogen III + 4 CO2Mutations and deficiency in this enzyme are known to cause familial porphyria cutanea tarda and hepatoerythropoietic porphyria.[5] At least 65 disease-causing mutations in this gene have been discovered.[6]
At low substrate concentrations, the reaction is believed to follow an ordered route, with the sequential removal of CO2 from the D, A, B, and C rings, whereas at higher substrate/enzyme levels a random route seems to be operative. The enzyme functions as a dimer in solution, and both the enzymes from human and tobacco have been crystallized and solved at good resolutions.

UroD is regarded as an unusual decarboxylase, since it performs decarboxylations without the intervention of any cofactors, unlike the vast majority of decarboxylases. Its mechanism has recently been proposed to proceed through substrate protonation by an arginine residue.[7] A 2008 report demonstrated that the uncatalyzed rate for UroD's reaction is 10−19s−1, so at pH 10 the rate acceleration of UroD relative to the uncatalyzed rate, i.e. catalytic proficiency, is the largest for any enzyme known, 6 x 1024M−1.[8]

|
PDB gallery
| |
|---|---|
|
1jph: Ile260Thr mutant of Human UroD, human uroporphyrinogen III decarboxylase
1jpi: Phe232Leu mutant of human UROD, human uroporphyrinogen III decarboxylase
1jpk: Gly156Asp mutant of Human UroD, human uroporphyrinogen III decarboxylase
1r3q: Uroporphyrinogen Decarboxylase in complex with coproporphyrinogen-I
1r3r: Uroporphyrinogen Decarboxylase with mutation D86N
1r3s: Uroporphyrinogen Decarboxylase single mutant D86G in complex with coproporphyrinogen-I
1r3t: Uroporphyrinogen Decarboxylase single mutant D86G in complex with coproporphyrinogen-III
1r3v: Uroporphyrinogen Decarboxylase single mutant D86E in complex with coproporphyrinogen-I
1r3w: Uroporphyrinogen Decarboxylase Y164F mutant in complex with coproporphyrinogen-III
1r3y: Uroporphyrinogen Decarboxylase in complex with coproporphyrinogen-III
1uro: UROPORPHYRINOGEN DECARBOXYLASE
|
|
| |||||||
|---|---|---|---|---|---|---|---|
| Porphyrin biosynthesis |
| ||||||
| Heme degradation tobile |
| ||||||
|
| |
|---|---|
| Activity |
|
| Regulation |
|
| Classification |
|
| Kinetics |
|
| Types |
|