


Inorganic chemistry, an ynone is an organic compound containing a ketone (>C=O) functional group and a C≡C triple bond. Many ynones are α,β-ynones, where the carbonyl and alkyne groups are conjugated. Capillin is a naturally occurring example. Some ynones are not conjugated.
One method for synthesizing ynones is the acyl substitution reaction of an alkynyldimethylaluminum with an acyl chloride. An alkynyldimethylaluminum compound is the reaction product of trimethylaluminum and a terminal alkyne.[1]

An alternative is the direct coupling of an acyl chloride with a terminal alkyne, using a copper-based nanocatalyst:[2]
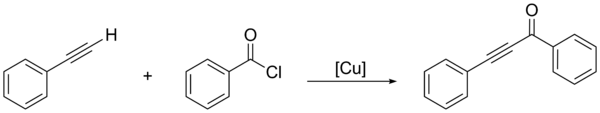
Other methods utilize an oxidative cleavage of an aldehyde, followed by reaction with a hypervalent alkynyl iodide, using a gold catalyst.[3]
An alternative but longer synthetic method involves the reaction of an alkynyllithium compound with an aldehyde. The reaction produces a secondary alcohol that then can be oxidized via the Swern oxidation.
Terminal alkynes add across α,β-unsaturated ketones in the presence of palladium catalysts. The reaction affords γ,δ-ynones.[4] Terminal alkynes add across epoxides to given yneols, which can be oxidized to give β,γ-ynones.[5]
{{cite journal}}: CS1 maint: multiple names: authors list (link)
{{cite journal}}: CS1 maint: multiple names: authors list (link)
|
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrocarbons (only C and H) |
| ||||||||||||||
| Only carbon, hydrogen, and oxygen (only C, H and O) |
| ||||||||||||||
| Only one element, not being carbon, hydrogen, or oxygen (one element, not C, H or O) |
| ||||||||||||||
| Other |
| ||||||||||||||
| |||||||||||||||